Amendment to Markush Claim in China and Takeaways
Date: 28 September 2023
【Volume 121】
A Markush claim is common in a patent in chemical or medical field. However, a Markush claim is highly generalized. For the balance between the certainty of a patent publication and the interests of a patentee, there are distinct opinions between each jurisdiction about the interpretation of a Markush claim and the legality of amendment wherein part of the elements in a Markush claim are deleted in a post-grant amendment stage. Here, we discuss the interpretation of a Markush claim and the related provisions about post-grant amendment in China by two cases, and provide some advises for corresponding strategies.
Markush claim in China: whole technical solution theory
There are two views in terms of the interpretation of a Markush claim in each jurisdiction: a “whole technical solution theory” and a “parallel technical solution theory”. In parallel technical solution theory, a Markush claim is interpreted as a collection of various specific compounds in parallel and therefore it is allowed to amend a Markush claim by deleting part of the elements in parallel. In whole technical solution theory, a Markush claim is interpreted as a generalized technical solution and therefore it is NOT allowed to amend a Markush claim by deleting.
In China, there is no specific description about the scope of a Markush claim in the Guidelines for Patent Examination in China. The courts in China held conflicting opinions about whether a Markush claim should be explained as a whole technical solution or a parallel technical solution in the precedent in the past.
Regarding this issue, the Supreme People’s Court in China revoked the judgment of the second instance in the case Daiichi Sankyo v Winsunny and established the principle of adopting whole technical solution theory for a Markush claim. The court clarified that a Markush claim was a generalized technical solution. Thereafter, Beijing Intellectual Property Court applied the same view in the case BASF v Shaanxi Meibang Pharmaceutical Group Co., Ltd..
The details are followed.
Case 1: Daiichi Sankyo v Winsunny(Supreme People’s Court No.41 Retrial Judgement of Administrative Litigation (2016))
1.Case facts
Winsunny Harmony Science & Technology Co., Ltd. (“Winsunny”) filed an invalidation action for the invention patent “Process for preparing pharmaceutical composition for treatment or prevention hypertension” (CN1121859C, ’859 patent) owned by Daiichi Sankyo Co., Ltd. (“Daiichi Sankyo”). In the invalidation proceeding, the patentee requested to amend the Claim 1 of ’859 patent. The Supreme People’s Court revoked the decision of the second instance in the end and held that a Markush claim is a generalized technical solution. The request for amendment was rejected.
’859 patent relates to a pharmaceutical composition for treatment or prevention hypertension, wherein the antihypertensive agent is at least one of the compounds with formula (I) or a pharmaceutically acceptable salt or ester thereof:
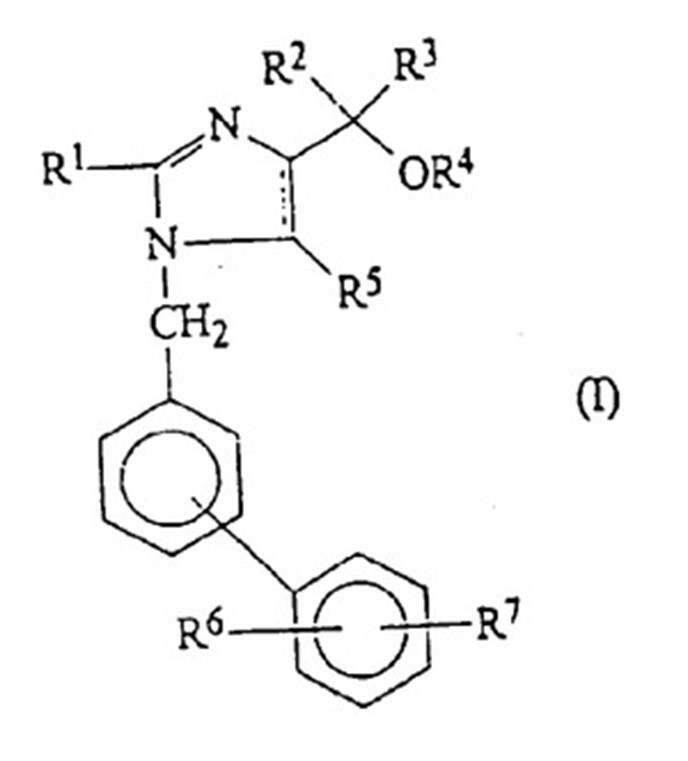
The Claim 1 of ’859 patent is described as a Markush claim limiting the R1~R7 in formula (I) to be selected from a group of substituents. Daiichi Sankyo requested for post-grant amendment to the Claim 1 which included deleting part of the substituents in the definition of R4 and R5 in the invalidation stage.
2.Opinion of the Supreme People’s Court
In a Markush claim, selecting different alternative options in the claimed group should generate pharmaceutical compositions with the same medical effect. That is, the pharmaceutical compositions including compounds of different molecular formulas should be interchangeable and achieve the same medical effect as expected. Therefore, the Markush Claim should be regarded as a collection of the Markush elements rather than a collection of individual compounds.
Also, when judging whether an amendment or post-grant amendment request for a Markush claim should be allowed, the principle should be that the amendment or post-grant amendment should not generate a group of or a single compound with new performance and effects. If a patentee is allowed to delete any option for any variable, even if the deletion reduces the scope of patent right without injuring public interest, whether the amendment generates new scope of patent right is uncertain, which leads to unstable public expectation and difficulty maintaining the stability of patent system.
The Supreme People’s Court in China established a clear and robust principle in this case, and clarified that a Markush claim should be interpreted with whole technical solution theory. The court considered that deleting part of the Markush elements would result in generating a technical solution with new combination generally which violates the regulations of amendments.
Case 2: BASF v Shaanxi Meibang Pharmaceutical Group Co., Ltd. (Beijing Intellectual Property Court No. 9342 First Trial Judgement of Administrative Litigation (2018))
1.Case facts
Shaanxi Meibang Pharmaceutical Group Co., Ltd. filed an invalidation action for the invention patent “Fungicidal mixtures” (CN1122442C, ’442 patent) owned by BASF.
The Claim 1 of ’442 patent described a fungicidal mixture comprising (a) a carbamate of the formula I.d with a weight ratio of 10∶1-0.01∶1
Accordingly, the main differences between the ‘761 application and the exhibits are that the biopolymer composition is slightly different, and the exhibits do not disclose the ratio of the contents.
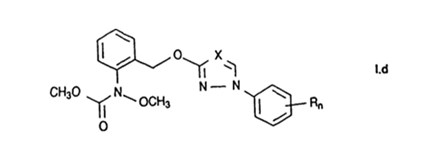
, wherein X is CH and N, n is 0, 1 or 2 and R is halogen, C1-C4-alkyl and C1-C4-haloalkyl, it being possible for the radicals R to be different if n is 2, or a salt or adduct thereof,
and (b) a fungicidally active compound from the class of the benzimidazoles or benzimidazole-releasing precursors (II), wherein the benzimidazoles or benzimidazole-releasing precursors (II) is selected from the group of compounds consisting of:
II.a: methyl 1-(butylcarbamoyl)benzimidazol-2-ylcarbamate
II.b: methyl benzimidazol-2-ylcarbamate
II.c: 2-(2-ethoxyethoxy)ethyl benzimidazol-2-ylcarbamate
II.f: dimethyl 4,4'-(o-phenylene)bis(3-thioallophanate)
BASF requested for deleting “X is N” in the description of “X is CH and N” in the Claim 1, that is, amending it into “X is CH”, in the Amendment 2.
2.Opinion of the Beijing Intellectual Property Court
The plaintiff argued that “X is CH” and “X is N” are two parallel technical solutions, so the amendment is a deletion of technical solution. However, the view of the plaintiff would only be accepted when a Markush claim is deemed as a collection of parallel technical solutions, that is, each of the Markush elements is an independent technical solution. Yet, a Markush claim should be deemed as one technical solution in principle, but not a collection of parallel technical solutions. Therefore, the request of the plaintiff to divide the Claim 1 into two parallel technical solutions based on the number of the options of X cannot be allowed. Correspondingly, the argument that deleting “X is N” is a deletion of technical solution cannot be accepted, either.
Apparently, this case followed the opinion of the Supreme People’s Court of Case 1 where it is emphasized that a Markush claim is a generalized technical solution. Accordingly, the view of Supreme People’s Court No.41 Retrial Judgement of Administrative Litigation (2016) has become a reliable opinion in China and the amendment to a Markush claim by a patentee has been greatly restricted thereafter.
Analysis of the opinion in China and Comparison with the opinion of EPO
In summary, China put a severe limitation on a post-grant amendment to a Markush claim. Amendment by deletion is only allowed when: (1) the amendment does not generate a group of or a single compound with new performance and effects; (2) the amendment does not lead to uncertainty of the scope of the claim, e.g., deleting any option for any variable would lead to uncertainty of the scope.
In fact, these opinions are similar to those of EPO. EPO stipulates the following limitations on an amendment by deletion in T 1506/13: first, the deletion must not result in singling out any hitherto not specifically mentioned individual compound or group of compounds, but maintains the remaining subject-matter as a generic group of compounds differing from the original group only by its smaller size; second, the deletion does not lead to a particular combination of a specific meaning which was not disclosed originally, i.e. it does not generate another invention.
Accordingly, the opinions in China about the amendment to a Markush claim are similar to those of EPO. However, China follows the principle more strictly. EPO focuses on the amendment must not “single out” specific compounds; in contrast, deleting any option for any variable in a Markush group may be considered as introducing new matter in China.
Wisdom Suggested Strategies
In practice, patentees often need to delete part of elements in a Markush group to meet the requirement of non-obviousness and support. However, the deletion may be considered to introduce new matter in China and therefore be rejected, which results in problems that the obviousness issue or lack of support cannot be overcome.
Therefore, patentees are suggested to describe the possible or preferred subsets with regards to various elements in Markush groups specifically as many as possible and list their specific examples when drafting the specification and claims, and also analyze the effect of changing the Markush elements on the performance of the compounds in the specification. It is also suggested to describe different combinations of different elements from larger to smaller scope in dependent claims and, furthermore, to list specific compounds which are actually used in parallel to increase the possibility of amending the Markush claims successfully based on the descriptions mentioned above in the subsequent proceedings.






