Invalidation of Compound “Upadacitinib” by CNIPA - Admissibility of Supplementary Experimental Data in China
Date: 19 January 2024
【Volume 127】
On December 20, 2022, SICHUAN GOWELL PHARMACEUTICAL CO., LTD. requested invalidation actions against two core patents of the drug, Upadacitinib, of AbbVie. China National Intellectual Property Administration (CNIPA) issued examination decisions No.561725 and No.562232 for invalidation requests on August 10, 2023 and declared that all claims of the compound patent of Upadacitinib, i.e., Invention Patent No. ZL201080062920.6 (‘920 patent), and part of its divisional patent, i.e., Invention Patent No. ZL201810902092.0 (‘092 patent, which is also the composition patent for Upadacitinib), were invalidated.
Upadacitinib is an oral selective JAK1 inhibitor developed by AbbVie and was granted marketing licenses by US FDA (with the brand name RINVOQ®) in August, 2019 and China National Medical Products Administration (NMPA) in February, 2022. In 2022, AbbVie reported that the global sales of Upadacitinib was up to US$2.52 billion and it was predicted that the sales would be over US$7.5 billion in2025. However, AbbVie may have to face competition from generic drug makers in China in advance due to the invalidation decision by CNIPA.
These two patents were kept valid in EU and US, wherein the EPO adopted the experimental data submitted by the patentee during the opposition procedure. However, CNIPA did not adopt the supplementary experimental data.
The following is the detailed analysis of the specific reasons for the invalidation decisions of CNIPA.
Main Technical Features of the Disputed Patents
AbbVie filed a post-granted amendment during the invalidation process to limit the claims to a specific compound, i.e., Upadacitinib (corresponding to the compound AA1.160 in the specification).
The amended Claim 1 of ‘920 patent:
A compound of following formula, or pharmaceutically acceptable salts thereof
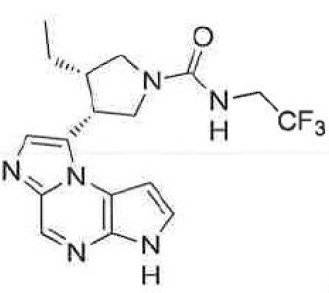
〔Chemical structure of Upadacitinib〕
Since Upadacitinib is not disclosed in the priority application US61/265563, the priority right cannot be claimed. AbbVie also agreed. Therefore, Exhibit 2 (WO2009/152133A1) can be used as prior art.
Requester’s Claim
1.Upadacitinib is a selection invention that falls within the scope of Claim 38 of Exhibit 2.
2.With Claim 38, the compound of Example 19, the compound of Example 9 and the compound of Example 16 in Exhibit 2 as the closest prior art, Upadacitinib is a compound with similar structure as the compound examples of Exhibit 2. Since the specification of ‘920 patent did not provide any technical effect related to Upadacitinib, combining Exhibit 2 and common knowledge, Upadacitinib is obvious.
Main Disputed Issue: Is Upadacitinib Obvious Compared to Exhibit 2?
1.AbbVie agreed that Upadacitinib falls within the scope of Markush Claim 38 of Exhibit 2:
Claim 38 of Exhibit 2 disclosed the formula (Ic) with the limits for the R1 to R5 groups.
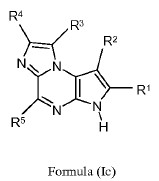
When R1, R2, R4, R5 are all H and R3 is ‑A‑D‑E‑G, wherein A, D, E, G are bond, ethyl-substituted pyrrolidine, ‑C(O)-NH-CH- and CF3 respectively, the compound of formula (Ic) is Upadacitinib.
2.Example 19, Example 9 and Example 16 in Exhibit 2 specifically disclosed the following compounds:
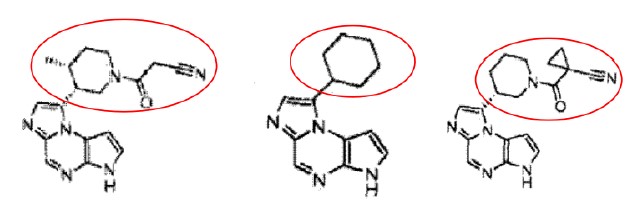
Compared to Upadacitinib, the lower part, the fused tricyclic ring, are identical and the difference lies in the groups of the upper part and Upadacitinib is in a stereoisomeric form. Their structures are similar with that of Upadacitinib.
Accordingly, the CNIPA indicated that investigation and comparison on the technical effects should be focused when determining whether Upadacitinib is obvious compared with Exhibit 2.
Opinion of the CNIPA
1.‘920 patent did not disclose the data showing the effect of Upadacitinib, so one can only conclude that Upadacitinib exhibits similar inhibitory activity with the disclosed compounds in Exhibit 2 based on the JAK3 inhibitory activity data of other specific compounds in the specification of ‘920 patent:
The CNIPA deemed that ‘920 patent is highly associated with Exhibit 2 with totally literally identical descriptions about technical background, purpose of invention and technical effects such as correlation between JAK kinases and indications. Also, the definition of formula of compound overlaps with formula (Ic) significantly, especially when the compounds of Example 19 and Example 16 in Exhibit 2 are the compounds AA1.27 and AA1.26 in ‘920 patent. However, Exhibit 2 did not provide any experiment result, while ‘920 patent disclosed JAK3 kinase inhibitory activity data (IC50 range) of 185 specific compounds (Upadacitinib was not included).
The CNIPA considered that in principle, when the claimed technical solutions are specific compounds, the specification shall present them with examples demonstrating preparation and/or effects to convict a person ordinarily skilled in the art that the invention has been completed prior to the time of filing.
However, the specification of ‘920 patent merely described the name, structural formula, general preparation method, retention time Rf, mass spectrometry data and other preparation-related information of Upadacitinib in tables without any experiment result of inhibitory activity. Therefore, a person ordinarily skilled in the art would anticipate at best that Upadacitinib has a JAK3 inhibitory effect similar to that of the tested 19 specific compounds with formula (Ic) in the specification of ‘920 patent, and that the compounds covered by the formula (Ic) of Exhibit 2 (including Upadacitinib) should also have roughly equivalent activity.
2.AbbVie submitted plenty of supplementary experimental data during the invalidation process to prove that Upadacitinib generates unexpected effects, which, however, were not adopted by the CNIPA:
Although AbbVie submitted Rebuttal Evidence 2 (Declaration of Expert) disclosing JAK3 inhibitory activity data (IC50) of 7 compounds including Upadacitinib, the CNIPA considered that the 6 compounds other than Upadacitinib were not in the scope defined by formula (Ic) in Exhibit 2, and therefore the experiment results cannot represent the difference of technical effects between Upadacitinib and that of the overall closest prior art.
Furthermore, Rebuttal Evidence 1, Rebuttal Evidence 2, Rebuttal Evidence 4 and Rebuttal Evidence 5 (Rebuttal Evidence 1 and Rebuttal Evidence 2 are Declarations of Expert, and Rebuttal Evidence 4 and Rebuttal Evidence 5 are the EPO opposition decision of ‘920 patent’s European equivalent and the related supplementary experimental exhibit submitted during the opposition procedure, respectively.) disclosed the Upadacitinib inhibitory activity data for JAK1, JAK2 and Upadacitinib selective inhibitory effect for JAK1/3. However, the CNIPA considered that ‘920 patent merely mentioned the mechanism, pharmacological experiment conditions and indications related to protein kinase inhibitory activity of several protein kinase inhibitors including JAK1, JAK2, JAK3, TYK2, KDR, Flt-2, wherein, except the kinase inhibitory activity of JAK3, the disclosure did not focus on the inhibitory activity and selective inhibition of other kinds of JAK kinase.
Therefore, the kinase activity data of JAK1 and JAK2 submitted by AbbVie and the selectivity deduced therefrom were beyond the teaching that a person ordinarily skilled in the art could possibly obtain based on the disclosure of the original patent filing. Thus, the supplementary experimental data was found to be inadmissible.
The CNIPA clearly verified their standpoint for supplementary experimental data. During defense stage, the patent applicants are allowed to submit supplementary experimental data after the filing date or priority date and the application may be examined based on the supplementary experimental data. However, it does not mean that the supplementary experimental data would definitely be adopted.
Admissibility of supplementary experimental data should be considered with relative restrictive criteria and the principle shall be that “the to be proved facts and/or technical effects which the supplementary experimental data can prove directly shall be able to obtained by a person ordinarily skilled in the art based on the disclosure of the patent filing documents”, that is, from the perspective of a person skilled in the art, supplementary experimental data serve as corroborative evidence of the effects which have been clearly and explicitly disclosed in the original filing documents, rather than a refocusing on the dozens of possible effects that are generally such documents.
In summary, due to the lack of evidence to prove better technical effects of Upadacitinib compared to the closest prior art, i.e., the compounds of formula (Ic) disclosed in Exhibit 2, or the specific compound disclosed in Example 19, Example 9 and Example 16, the CNIPA deemed that the technical problem actually solved by Upadacitinib is to merely provide a compound with a structure similar to that of the compound as disclosed by Exhibit 2. Therefore, on the basis that Exhibit 2 has provided clear teachings for the definitions of substitution groups, a person ordinarily skilled in the art should be able to obtain Upadacitinib easily. In conclusion, it was determined that the ‘920 patent was obvious.
Wisdom Suggested Strategies
Regarding whether the supplementary experimental data submitted after the filing date shall be adopted, the section 3.5 in the 2021 version of “Guidelines for Patent Examination in China” stipulates that “Supplemental experimental data submitted after the date of filing by the applicant in order to meet the requirements of Articles 22.3, 26.3, etc. shall be examined. The technical effect proven by the supplemental experimental data shall be an effect which can be determined from the disclosure of the application by a person skilled in the art.” and two specific examples are further added in the section 3.5.2. 1
The CNIPA clearly verified the judging principle that “the to be proved facts and/or technical effects which the supplementary experimental data can prove directly shall be able to be obtained by a person ordinarily skilled in the art based on the disclosure of the patent filing documents” again in the above two invalidation decisions, that is, supplementary experimental data serve as corroborative evidence of the effects which have been clearly and explicitly disclosed in the original filing documents, rather than a refocusing on dozens of possible effects that are generally disclosed in such documents. According to the invalidation decisions, for specific compounds, specification shall disclose experimental data for proving the effect of compounds sufficiently, to enhance the admissibility for supplementary experimental data that were submitted later.
In fact, compound patent of Upadacitinib faced opposition in Europe as well. However, the EPO adopted the supplementary experimental data provided by AbbVie and determined that Upadacitinib was inventive compared to WO2009/152133A1 (i.e., Exhibit 2) on the basis of the experimental results and kept the patent valid. Accordingly, it is noted that the U.S. and Europe hold more open attitude regarding supplementary experimental data and prefer to find technical facts by supplementary experimental data, wherein supplementary experimental data can be used to prove the technical effects mentioned in specification without specifically disclosed experimental data and clarify the related effects indicated or implied in the patent and the difference with prior art.
In addition, these two invalidation decisions also relate to the determination of priority. According to the stipulation of “Paris Convention” and Article 29 of “Patent Law of the People's Republic of China”, priority is established to provide the procedural interest for applicant to file applications in different countries in a limited period, rather than providing extra period to complete the substantial content of the invention. Patentee can still complete or supplement content of the invention during the period of priority, while this comes with the risk of that the priority right cannot be claimed for the added content. If the priority documents include only general research idea, it should not be deemed as an invention sharing the same subject with an application filed later in which a specific technical effect is clearly evaluated. In addition, if the original claim is a Markush claim, an applicant may delete or further limit the definition of some groups to form a Markush claim with smaller scope, provided that the deletion or limitation does not generate specific combination with specific meaning or emphasize single compound or a group of compounds which is not specifically mentioned in the original filing documents. Meanwhile, the amendment is still within the whole scope of the Markush claim and does not generate new core of invention or changes in the invention substantially, either. In this case, it can be deemed that the invention has the same subject as the original invention and is allowed to claim priority right of the application filed earlier.
[1]For more information about supplementary experimental data, please refer to Wisdom News Vol. 54 (https://www.wisdomlaw.com.tw/m/405-1596-104026,c12252.php?Lang=en)






