Wisdom Secures Another Win in Pharma Patent Litigation
Date:11 August 2023
【Volume 115】
In June 2023, the Taiwan Intellectual Property Court (IP Court) issued a second-instance judgment in a patent linkage litigation. The first dismissal decision of the case marked the first successful challenge by a generic drug company that filed a Paragraph IV declaration against a brand drug company. (Bayer HealthCare LLC v Synmosa Biopharma Corporation, IP Court 2022 Minzhuanshangzi No. 6 Judgment). The Court of Second Instance upheld the first-instance judgment1 and dismissed Bayer's appeal. Wisdom has once again successfully represented and assisted the appellee, Synmosa Biopharma Corporation, in obtaining a complete victory in the second instance. Wisdom Director George J. H. Huang (Attorney at Law, Patent Attorney) led the team representing Synmosa.
Case Facts
This case pertains to a dispute involving the generic drug of Sorafenib. In the first instance, Synmosa argued that all the claims (a total of 26) of Bayer's polymorph patent of sorafenib tosylate (Certificate No. I382016) and pharmaceutical composition patent thereof (Certificate No. I324928) were invalid, which was recognized by the Court of First Instance. During the appeal proceedings, Bayer requested the summoning of its expert witness to testify, and Synmosa likewise sought the summoning of its own expert witness. Consequently, the court scheduled a single hearing date for both expert witnesses and allowed the lawyers of both parties to cross-examine them. However, in the end, the court only accepted the testimony of Synmosa's expert witness. The Court of Second Instance provided a detailed analysis in its judgment regarding the qualification and credibility of the expert witnesses, which is highly informative. The Court of Second Instance ultimately upheld the invalidity of all the claims (a total of 26) of Bayer's patents and dismissed Bayer's appeal.
Furthermore, the Court of Second Instance provided a detailed elaboration on the determination of inventiveness for polymorph compounds, motivation to combine in pharmaceutical patents, etc., which holds significant importance and value for reference. Below, we will present the key points of the second instance's judgment.
Main Technical Features of Bayer's Patents
The two patents concerned are the polymorph patent of sorafenib tosylate (Certificate No.: I382016, “‘016 patent”) and the pharmaceutical composition patent thereof (Certificate No.: I324928, “‘928 patent”).
1. ‘016 patent:
(1) The technical features of claim 1 of ‘016 patent include:
(1A) A compound of the formula (I) (sorafenib tosylate)
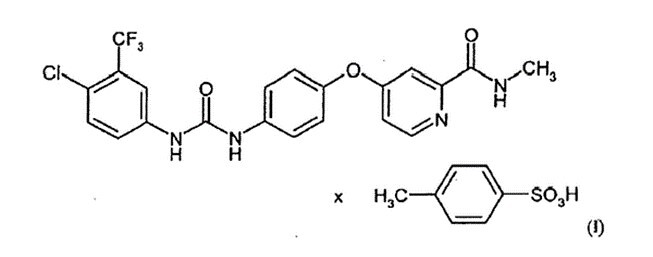
(1B) in the polymorph I form, which shows in the X-ray diffractometry peak maxima of the 2 Theta angle of 4.4, 13.2, 14.8, 16.7, 17.9, 20.1, 20.5, 20.8, 21.5 and 22.9.
(2) Claims 2-3 of ‘016 patent contain additional limitations that the claimed polymorph I shows specific peak in the IR spectrum and Raman spectrum.
(3) Claims 4-6 of ‘016 patent relate to the process for preparing the polymorph I of claim 1, which recite different recrystallization methods, specifically by inert solvent (claims 4-5), melt crystallization (claim 6, which defines specific heating and cooling conditions), to convert the compound of formula (I) in the polymorph II form to the polymorph I form.
(4) Claims 7-15 of ‘016 patent are directed to the medical use and the pharmaceutical composition of the polymorph I in claim 1.
2.‘928 patent:
(1) Claim 1 of ‘928 patent is directed to a pharmaceutical composition, having the following technical features:
(1A) active agent in a portion of at least 55% by weight of the composition (drug load feature);
(1B) said active agent is sorafenib tosylate (active agent feature);
(1C) at least one pharmaceutically acceptable excipient selected from the group consisting of filler, disintegrant, binder, lubricant, and surfactant (excipient feature);
(1D) the composition is a tablet (dosage form feature).
(2) Claim 2 of ‘928 patent relates to further limitation on the feature 1A, where the active agent present in the composition is at least 75% by weight; claim 3 relates to additional limitation on feature 1B, where sorafenib tosylate exists for at least 80% in the stable polymorph I; claims 4-5 relate to additional limitation on the feature 1C, where the claimed composition comprises the excipients in specific types, and in specific amounts; claim 6 relates to further limitation on the feature 1D, where the tablet is an immediate release tablet; claims 7-8 relate to further limitation on the feature 1B, where the active agent is micronized and with a particle size from 0.5 to 10 micrometer.
(3) Claims 9-11 of ‘928 patent further define the water content of the claimed composition, as well as the combination with other therapeutic agents and the medical use.
The Second Instance Court Opinion
1. The determination of whether the testimony of expert witnesses should be accepted:
Bayer requested the appearance of Professor Jia-You Fang as an expert witness, while Synmosa requested the appearance of Professor Shan-Yang Lin as their expert witness. Both expert witnesses had issued expert reports. The court decided to summon both expert witnesses for cross-examination on the same hearing date, during which the lawyers from both sides conducted three hours of cross-examination. However, in the end, the court only relied on the testimony of Professor Shan-Yang Lin, Synmosa's expert witness.
In the judgment, the court provided a detailed explanation regarding the difference in admissibility between the testimonies of the two expert witnesses. The key points are summarized as follows:
(1)、The qualifications of the witnesses could affect the credibility of their testimonies:
The court observed that Professor Jia-You Fang is not a practitioner in the pharmaceutical industry, and his area of expertise does not specifically pertain to matters concerning pharmaceutical polymorphs. Therefore, there may be doubts about whether his opinions truly represent the technical procedures used in the tablet manufacturing sector. In contrast, Professor Shan-Yang Lin's research focuses on cocrystal, drug-excipient interactions, changes in pharmaceutical polymorphs, etc. In addition, his testimony regarding the necessity of pre-formulation studies on polymorphs during drug development is consistent with the significance emphasized in Synmosa Exhibit 2 regarding controlling the crystal form of the active pharmaceutical ingredient (API).
(2)、Even if an expert witness is an experienced senior in the industry, as long as the content of their testimony corresponds to common knowledge, they are qualified as a person having ordinary skill in the art (PHOSITA):
Bayer argued that Professor Shan-Yang Lin's testimony is not representative of a PHOSITA because he is an expert in the pharmaceutical industry and has expertise beyond that of a PHOSITA.
Nonetheless, the court found that Professor Shan-Yang Lin's testimony aligns with the technical content recorded in Synmosa Exhibits 49 and 50. Since the publication dates of Synmosa Exhibits 49 and 50 are earlier than the patent at issue's priority date of 2005, it indicates that "as of 2005," a PHOSITA would have the motivation to study and achieve the most stable crystal form of a drug. Therefore, Professor Shan-Yang Lin's testimony is consistent with the knowledge of a PHOSITA before 2005.
(3)、If an expert witness's testimony lacks supporting evidence from other technical documents or contradicts other evidence, it should not be accepted:
The court held that although Professor Jia-You Fang claimed that a PHOSITA would not immediately analyze polymorphs when producing pharmaceuticals, his testimony lacked supporting evidence and therefore raised doubts. Furthermore, his testimony did not completely rule out the possibility that a PHOSITA might proactively test the stability of polymorphs during pharmaceutical production.
Additionally, although Professor Jia-You Fang stated that tosylate salts are a very rare form of drug salt and cannot be prepared from the preparation steps of a compound, thereby rendering them unobtainable through routine experimentation by a PHOSITA. Conversely, the court found that the specification of the ‘016 patent already mentioned in the "Prior Art" section that "the compound of the formula (I) is prepared according to a general standard method for the preparation of tosylate salts." As the patent owner (Bayer) is a major player in the industry, the prior art section of their specification should be the most accurate representation of the actual situation in the pharmaceutical tablet manufacturing field. Thus, it becomes even more difficult to argue that practitioners in that field would be unable to prepare it through routine experimentation.
2. Regarding the existence of the motivation to combine multiple pieces of evidence:
Bayer argued that the first instance erroneously presumed the existence of a motivation to combine solely based on the single factor of technical relevance between the evidence without considering other factors such as commonality in the problems to be solved. They contended that this conclusion clearly contradicts the law.
However, the court believes that the commonality of the problems to be solved, the commonality of functions and effects, and the teachings or suggestions among multiple pieces of evidence should be determined based on the substantive technical content implied in those pieces of evidence, including what can be readily conceived by those skilled in the art based on such evidence. Synmosa Exhibit 1 not only discloses sorafenib tosylate as claimed in the '016 patent but also provides teachings for the preparation of tablets using this compound. Those skilled in the art could easily conceive that in the process of formulating pharmaceuticals, there would be issues concerning the weight of the active ingredient and the total weight of the corresponding tablets. Moreover, Synmosa Exhibit 10 discloses the technical content for solving the aforementioned issue. Therefore, the first instance judgment has substantially considered the commonality of the issues, teachings, or suggestions among the aforementioned evidence as a motivation for combination.
3. The thermodynamically stable polymorph of the API also possesses mechanical stress stability, which is not unexpected:
Bayer argues that the mechanical stress stability (a short-term phenomenon) cannot be inferred from the thermodynamic stability (a long-term phenomenon) of the polymorph, and that polymorph I of Sorafenib tosylate demonstrates both thermodynamic stability and mechanical stress stability, along with sufficient solubility, resulting in unexpected effects. Therefore, Bayer contends that the conclusion of the first trial is erroneous.
However, this argument was not accepted by the court. First, the court observed that the patent specification or presented test data does not provide a specific and objective definition of "mechanical stress stability," and the test data provided by Bayer does not conclusively establish the mechanical stress stability of the polymorph claimed in '016 patent. Second, Bayer Exhibit 18 discloses that under "ordinary" circumstances, grinding will transform the metastable polymorph of the API into a stable polymorph. Thus, it is generally understood by a PHOSITA that most thermodynamically stable polymorphs of API also exhibit mechanical stress stability. While Bayer Exhibit 18 also mentions exceptions, it does not render the characteristic of "thermodynamically stable polymorph of the API also possessing mechanical stress stability" unexpected.
4. A person skilled in the pharmaceutical field can readily prepare the salt of a compound using common knowledge, provided they can produce the compound according to the specification:
Bayer argued that merely disclosing the name of a compound does not indicate that the compound has been disclosed in the prior art, and a PHOSITA cannot learn how to produce or use Sorafenib tosylate from Synmosa Exhibit 1. They also relied on the report and testimony of Professor Jia-You Fang to assert that tosylate salts are a relatively rare form of drug salt, and currently, the FDA has only approved nine drug using tosylate salts.
However, the court held that:
(1) Typically, the claims in pharmaceutical compound patents are directed to "a... compound and salts thereof," and the specification thereof usually only includes examples of the compound's preparation without specific examples of its salt form's preparation. Therefore, for a PHOSITA, as long as they can produce a compound based on the specification, they can readily prepare the salt of that compound using common knowledge. Since Synmosa Exhibit 1 has already disclosed the preparation process of Sorafenib with the disclosure of its counterion with tosylate salt, a PHOSITA should be able to prepare Sorafenib tosylate using common preparation methods.
(2) The evaluation of drug approval by the FDA and the examination of patents are two distinct matters. The relative scarcity of FDA-approved tosylate salt drugs does not necessarily relate to technical difficulties in their preparation.
5. Bayer has not demonstrated that the parametric feature of drug loading in '928 Patent has unexpected effects:
Bayer argued that the technical feature of drug loading defined in '928 Patent as being above 55% can achieve unexpected effects, such as hardness greater than 100N and almost 100% drug release after 15 minutes, and that commercially available high-loading drugs are very rare, as shown in Bayer Exhibit 58.
However, the court did not accept Bayer's argument. The court observed that while '928 Patent discloses an example with a drug loading of 80%, it does not provide a comparative example with Sorafenib content below 55%. Thus, it cannot be determined whether the effects presented in that example are due to the distinct technical feature. Moreover, '928 Patent does not provide information on the unexpected effects of Sorafenib tablets with a drug loading above 55% as compared to Sorafenib tablets with a drug loading below 55% under the same tablet formulation conditions.
Wisdom Suggested Strategies
In this case, Bayer's main strategy during the appeal stage is to challenge the findings of the first-instance court through expert witness testimony.
In the current practice of civil cases in the Taiwan Intellectual Property Court, it is not common for both parties to summon expert witnesses, as judges tend to rely more in written evidence than witness testimonies when making decisions. Therefore, this case can be considered a highly classic example. In fact, during the later stages of the first-instance trial, Bayer also submitted an expert witness opinion from a German researcher employed by Bayer Germany and requested for his appearance in court. However, the judge deemed the claims in the expert witness opinion was not credible and rejected Bayer’s request.
During the appeal stage of this case, Bayer sought to engage another expert witness, Professor Fang, a Taiwanese professor from Taipei Medical University, to testify. While the court accepted the application, it concluded that Professor Fang’s expertise, being in a different field from the patent at issue, lacked supporting evidence from other technical documents and even contradicted scientific theories, leading to a lack of trust in his testimony. On the contrary, the court fully relied on the testimony of Professor Lin, the witness of Synmosa represented by our firm, as his expertise was in the same field as the patent at issue, and his testimony was supported by other technical documents.
In the current practice of pharmaceutical patent litigation in Taiwan, it is foreseeable that the use of expert witness opinions or the application for their appearance in court as evidence will increase. Parties desiring to utilize expert witness testimony as evidence, whether as a drug patentee or a generic drug manufacturer, must pay special attention to the guidance provided by this judgment on the acceptance or rejection of expert witness testimony, in order to achieve a successful outcome.
[1] For an introduction to the first-instance judgment, please refer to https://www.wisdomlaw.com.tw/m/405-1596-106276,c12252.php?Lang=en






