PART I— Five Patent Invalidation Challenges- CSPC Succeeded in invalidating Mitsubishi Tanabe Pharma Corporation’s Novastan® HI injection 10 mg/2mL Patent
Date: 25 October 2022
【Volume 94】
The China National Intellectual Property Administration (CNIPA) issued Patent Invalidation Decision No. 56884 on June 29, 2022, declaring all claims of the invention patent “Pharmaceutical preparations containing arginine amides” (CN200480007612.8) (“the ‘612 patent”) corresponding to Novastan® HI injection 10 mg/2mL held by Mitsubishi Tanabe Pharma Corporation are invalid.
The ‘612 patent is a patent related a new pharmaceutical preparation patent of the active ingredient Argatroban, which aims to improve the convenience of operability, safety and stability of the known dosage form. According to the examination records of the CNIPA, at least 4 requests for invalidation of the ‘612 patent had been filed since December 2021, which showed that this improved technology may potentially generate immense commercial interests for pharmaceutical companies.
In the process of judging inventive step (obviousness) of the invention, the CNIPA complied with the principle and standard for determination of “three-step method” to make an insightful analysis. In the “three-step method,” the objective identification of the technical problem solved by the invention is an important prerequisite for the correct conclusion of the subsequent obviousness analysis. In practice, patentees usually claim the technical problem actually solved by means of multiple technical effects disclosed in the specification, but the multiple technical effects disclosed in the specification do not necessarily mean that the invention actually solves the corresponding technical problem compared to the closest prior art. Rather, the technical problem should be determined based on the fact that the technical effects of the invention can be achieved by the distinguishing technical features. We will introduce and analyze the key points of this case as follows:
Requirements of the China Patent Examination Guidelines
Examination practice of inventive step in China adopts the “three-step method” as stipulated in the Patent Examination Guidelines, which mainly includes:
i. Determining the closest prior art
ii. Determining the distinguishing technical features of the invention and the technical problem actually solved by the invention
iii. Determining whether or not the claimed invention is obvious from the closest prior art and the technical problem actually solved by the invention.
The technical problem actually solved by the invention is the technical task requires to improve the closest prior art in order to obtain better technical effects.
Whether the invention is obvious or not should depend on whether there is certain technical teaching in the prior art as a whole, that is, whether the prior art provides a teaching to apply the distinguishing technical feature to the closest prior art to solve the above-mentioned technical problem, and that this teaching would motivate person having ordinary skill in the art to improve the closest prior art and obtain the claimed invention when he is confronted with the described technical problem.
Main Technical Feature of the ‘612 patent
The ‘612 patent provides the novel Argatroban injection having the following technical features (Claim 1):
(1) The concentration of Argatroban ranges from 1 mg/ml to 10 mg/ml;
(2) the composition of the solubilizer: the mass/volume percentage of monohydric alcohol is 25%, the mass/volume percentage of dibasic alcohol or trihydric alcohol is 40%~55%, and is free from sugars.
The ‘612 patent provides an improved Argatroban injection. According to the description in the specification and the testimony of the expert witness, HIROKAWA, TAKESHI (who is also the inventor of the ‘612 patent), in this case, it is known that Argatroban is water-insoluble, and the specification for preparations on the market in Japan in 1960 was 10 mg:20 ml ampoule. However, the drug was used for new indications afterwards, and the dosage of the drug was greatly increased. For a 20ml ampoule for large-volume, it is time-consuming to draw the solution from an ampoule into a syringe, so there is an urgent need to reduce the amount of stock solution (water content) and increase the content of Argatroban in the single unit preparation to provide a convenient preparation.
In fact, Pfizer has developed the Argatroban injection 250 mg:2.5 ml by using the high concentration of ethanol and sugar as the solubilizer to dissolve 250 mg of Argatroban, but the high concentration of ethanol would lead to adverse effects such as inhibition of vasodilatation or central action. Moreover, the testimony of the expert also pointed out the following problems with the Pfizer’s Argatroban injection: (1) crystallization and precipitation at a low temperature; (2) high viscosity of pharmaceutical agent due to high concentration and unsuitable excipients, which makes it difficult to operate when using a syringe to draw up and dispense the drug.
Decision of CNIPA
I.The Closest Prior Art
The CNIPA found that US5214052A (“the ‘052 patent”) was the closest prior art.
The patentee claimed that: the Argatroban preparation in the ‘612 patent exclude the use of sugar, but the ‘052 patent disclose that the Argatroban preparation must use sugar; thus, the ‘052 patent cannot be deemed as the closest prior art. In addition, the patentee emphasized that the starting point for modification of the ‘612 patent was the Argatroban injection 10mg:20ml.
However, the CNIPA deemed that the ‘052 patent clearly disclosed that Argatroban is dissolved in a mixture of ethanol and glycerol, which described that “Arginineamides and/or a salt thereof may be used in a mixture of ethanol and glycerol, and sugar may be mixed with a solvent of alcohol and water; if necessary, any sugar may be mixed with a solvent of alcohol and water, and the obtained pharmaceutical composition may be used as an injection and for the treatment of thrombosis.”.
Apparently, the ‘052 patent does not teach that sugar is required for Argatroban preparations.
In addition, page 11 of the ‘612 patent specification, clearly discloses that “sugar may also be used in the pharmaceutical preparations or pharmaceutical compositions of the present invention...Since the use of sugar may sometimes increase the viscosity of the solution, it is generally not preferable to add a large amount of sugar.”. According to the ‘612 patent and the testimony of expert witness, the pharmaceutical composition of the ‘612 patent is used for the treatment of thrombosis.
It is obvious that the ‘612 patent only states that using a large amount of sugar would lead to excessively high viscosity of injection and so the preparation containing sugars is not a preferable selection. However, the ‘612 patent does not completely exclude the use of sugars.
Although the patentee emphasized that the 10mg:20ml Argatroban injection shall be deemed as the starting point of improvement of the ‘612 patent, the CNIPA pointed out that the starting point of improvement in the experimental study and the closest prior art selected in the patent examination are not necessarily related. Thus, even though the actual starting point of improvement in the ‘612 patent is different from the ‘052 patent, it does not affect the ‘052 patent being the closest prior art.
In summary, the CNIPA found that the ‘052 patent does not teach away from the ‘612 patent, and they are both pharmaceutical compositions for the treatment of thrombosis; thus, the ‘052 patent can be the closest prior art, wherein Example 6 in the ‘052 patent can be the closest technical means.
II.The Distinguishing Technical Features
Example 6 in the ‘052 patent discloses the pharmaceutical composition for injection, which is calculated to contain 100 mg/ml Argatroban, and the mass/volume percentage of ethanol is 20%, the mass/volume percentage of glycerol is 20%, and the mass/volume percentage of sorbitol is 30%.
Thus, the distinguishing features of the ‘612 patent as compared to Example 6 in the ‘052 patent are:
(1)The concentration of Argatroban is different: the ‘612 patent is 1 mg/ml~10 mg/ml, the ‘052 patent is 100 mg/ml;
(2) The composition of the solubilizer is different: in the ‘612 patent, the mass/volume percentage of monohydric alcohol is 25%, the mass/volume percentage of dibasic alcohol or trihydric alcohol is 40%~55%, and is free from sugars; in the ‘052 patent, the mass/volume percentage of monohydric alcohol, ethanol, is 20%, the mass/volume percentage of trihydric alcohol, glycerol, is 20%, and contains the sugar, sorbitol.
III.The Technical Problem Actually Solved by the Invention
As compared to the ‘052 patent, the patentee claimed that the technical effects of the ‘612 patent are “pharmaceutical convenience” and “stability”, wherein “pharmaceutical convenience” refers to the convenience of injection and suction, the convenience of dispensing, the reduction of the amount of stock solution, and the maintenance of a higher content of active ingredients; “stability” refers to the stability of crystallization and precipitation at a low temperature.
However, the CNIPA did not accept the above claim, and considered that technical problem actually solved by the ‘612 patent was to provide a different specification of the Argatroban pharmaceutical composition having a suitable viscosity for injection.
1.Pharmaceutical convenience
According to the specification of the ‘612 patent, it is known that the Argatroban pharmaceutical composition of the ‘612 patent has a specific viscosity that can be easily drawn up into the syringe, and the operability will be reduced by using a syringe needle which is thinner than 21G. The viscosity of the pharmaceutical composition is 10 millipascal seconds (mPa·s) or less, and preferably as low as possible; in addition, the use of a high-viscosity solution with a viscosity of 20 mPa·s or more may cause some problems such as difficulty in filling the correct volume in the dispensing process to the ampoule during manufacturing.
Accordingly, the CNIPA deemed that it cannot be inferred that the selection of the type and concentration of the solubilizer of the ‘612 patent can achieve the most preferred suction convenience from the specification, the details as described below:
(1) The pharmaceutical convenience in the ‘612 patent actually refers to the convenience of suction. The viscosities disclosed in Tables 1~11 are all related to the convenience of suction, and the convenience of dispensing is only mentioned in the comparative example. Moreover, the ‘612 patent discloses that the problem about the convenience of dispensing would be encountered only when the viscosity is 20 mPa·s or more, but the range of viscosity claimed in the ‘612 patent is 15 mPa·s or 10 mPa·s or less. Thus, the problem about the convenience of dispensing does not exist, and the convenience of suction and dispensing are both related to the viscosity of the preparation.
(2) Other preparations, despite not having the technical features as defined in the claim of the ‘612 patent, still have the viscosity equivalent to the claimed preparations of the ‘612 patent. It was found that the preparations commercially available in the U.S. contained sugars in the comparative example, and neither ethanol nor glycerin fell within the scope of the ‘612 patent, but their viscosities were 13.8 mPa·s which also met the viscosities required for the convenience of suction. In addition, referring to Table 3 of the ‘612 patent, in comparison with the pharmaceutical compositions 9 and 10, although pharmaceutical compositions 11 and 14 do not fall within the scope of claim 2, they are still within the range of viscosity required for the convenience of suction as claimed in the ‘612 patent.
〔Table 3 in the ‘612 patent〕
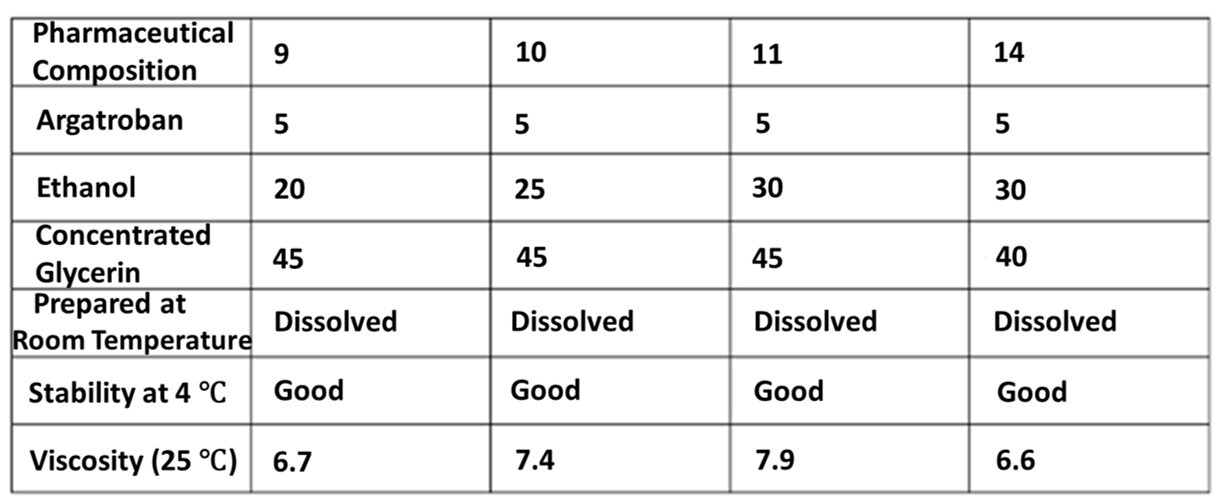
(3) The viscosity of the pharmaceutical composition is mainly determined by the solubilizer and is irrelevant to the content of Argatroban.
(4) The pharmaceutical composition 18 (refer to Table 5 in the ‘612 patent) contains 10% ethanol, 29.5% glycerol and 20% sorbitol by the mass/volume percentage. Although the solubilizer contains sugar, its viscosity is 6.65 mPa·s, which is still within the range of viscosity that can ensure the convenience of suction.
In the next part, the CNIPA’s opinions concerning determination of the technical problems actually solved by the invention and the obviousness of the technical means will be introduced in detail. Also, our comments and strategies will be provided.






