CNIPA Made Landmark Decisions of Inventiveness for Crystal Form Patents
Date: 5 July, 2024
【Volume 138】
Recently, there is an intensively increasing amount of crystal form invention patents for drugs. However, litigations related to crystal form patents are hardly settled before the drugs enter the market due to the fact that the crystal form patents are not included in the patent linkage system in China. Hence, even for the generic drug makers who challenged a patented drug successfully and are granted a 12-month market exclusivity period, they may still be unable to market their drugs because the generic drugs infringes the crystal form patents. Therefore, invalidation requests for crystal form patents become more important nowadays. Vortioxetine is the active substance of antidepressant medicine “Brintellix®.” Since 2018, the compound patent (ZL02819025.4) and the crystal form patent (ZL 200780022338.5) have respectively survived several patent invalidation challenges in China. The China National Intellectual Property Administration (CNIPA) made landmark determinations of the clarity requirement and inventiveness requirement for crystal form patents in the invalidation challenge decisions, No. 48337 decision and No. 48339 decision, of the crystal form patent.
The main technical features of the disputed patent
The disputed patent (ZL 200780022338.5) with the title “1- [2- (2, 4-dimethylphenylsulfanyl) -phenyl] piperazine as a compound with combined serotonin reuptake, 5-HT3 and 5-HT1A activity for the treatment of cognitive impairment” claims β-type crystal form of hydrobromide of Vortioxetine, a composition comprising thereof, auxiliary materials proportions for a formulation and a use of treating neurological diseases.
The drug sold on the market is the hydrobromide with the following structure:
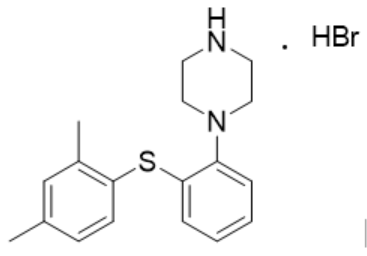
The specification of the disputed patent described different types of crystal forms of various salts with a total of 17 types of crystal forms, including crystal forms of hydrobromide, crystal forms of hydrochloride, and the melting point, hygroscopicity and solubility of these crystal forms.
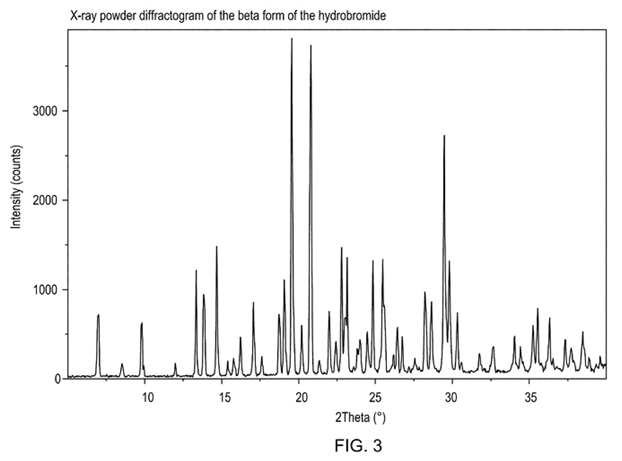
Claim 1: A hydrobromide of compound 1-[2-(2,4-dimethylphenylsulfanyl)-phenyl]piperazine in a crystal form characterized by an XRPD pattern as shown in FIG. 3.
Opinions of CNIPA
1. Clarity requirement:
Claim 1 of the disputed patent included the XRPD pattern of FIG. 3 as a limiting technical feature. However, the invalidation requester claimed that there are 30 or more characteristic peaks in the XRPD pattern of FIG. 3, but the specification only specifically described 4 main characteristic peaks without specifically disclosing the number of characteristic peaks and their values. Therefore, claim 1 is unclear.
However, the examiner thought that it was usually difficult to label all the diffraction peaks one by one with their intensities and locations or describe them with words, and it was common to define a crystal form with selected main characteristic peaks. The specification of the disputed patent had disclosed the main characteristic peaks at 6.89, 9.73, 13.78 and 14.62(°2θ) of the pattern. Therefore, a person ordinarily skilled in the art could clearly understand the β-type crystal form of hydrobromide claimed in claim 1 according to the XRPD pattern of FIG. 3 and the main characteristic peak, and could distinguish the β-type crystal form of hydrobromide and other known crystal forms according to the disclosure of the specification. Therefore, claim 1 met the clarity requirement.
2. Inventiveness requirement:
The prior art disclosed the free base and pharmaceutically acceptable acid addition salts of Vortioxetine. However, it failed to disclose their physical and chemical properties. The examiner thought that when judging unexpected technical effects, not only the differences between the different salts, but also the different effects of different crystal forms of the same salt had to be taken into consideration. The examples in the specification of the disputed patent had disclosed the melting point, hygroscopicity and solubility data of the free base and 9 types of crystal forms of salts of Vortioxetine, including 5 types of crystal forms of hydrobromide. The following is a summary of disclosure about some crystal forms:
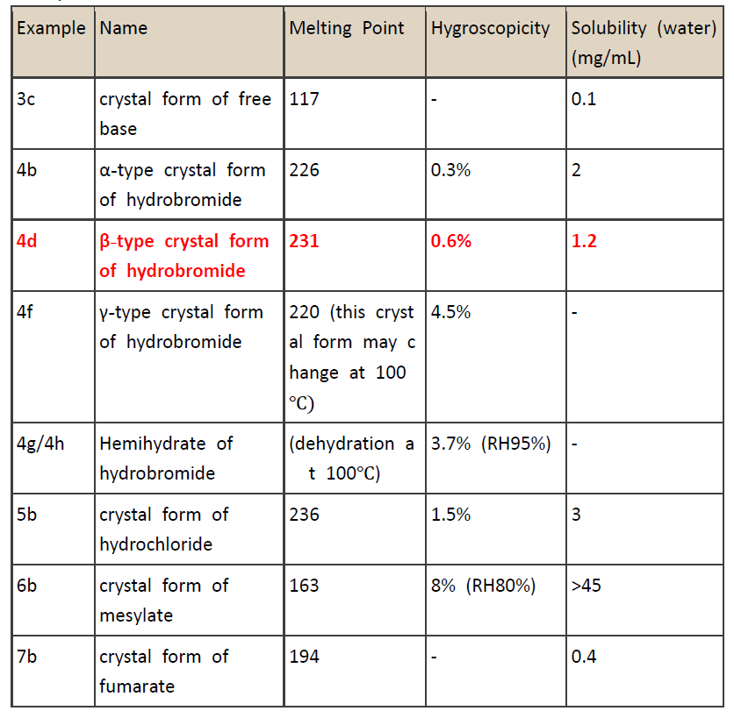
The β-type crystal form of hydrobromide of Vortioxetine has a higher melting point (which represented higher stability) compared with other salt forms and other crystal forms of hydrobromide. Meanwhile, it also exhibited relatively lower hygroscopicity, and higher solubility compared with other salt forms and other crystal forms of hydrobromide. The β-type crystal form of hydrobromide with low hygroscopicity and appropriate solubility was especially suitable for preparing oral tablets. Generally, forming salts from compounds helps increase solubility, while forming crystals from these salts tends to reduce their solubility to a certain extent. However, crystal forms have higher melting points, which indicates crystal forms have aligned microstructure and therefore have better stability and lower hygroscopicity. Therefore, the crystal form claimed in the disputed patent with a combination of technical effects of “high melting point, low hygroscopicity, and appropriate solubility” achieved a desired “balance” between these criteria of properties. By contrast, Exhibit 6, Exhibit 7, Exhibit 8 all failed to provide related teaching or suggestion about how hydrobromide of a medical compound and a specific crystal form thereof can achieve the combined effect that “all of the stability, solubility, and hygroscopicity are in an appropriate range.” Therefore, the claimed crystal form achieved unexpected technical effects and had an inventive step.
In an examination of an invalidation case for a crystal form patent, inventiveness remains to be the most important factor when judging whether the crystal form patent should be invalidated. Further, whether it has an “unexpected technical effect” is the key to the inventiveness of the crystal form patent.
In the Vortioxetine invalidation cases, the unexpected technical effect is the combined effect of improving multiple properties including appropriate stability, solubility, and hygroscopicity of the new crystal form. Therefore, the technical effects of the new crystal form in multiple aspects, such as physical/chemical stability, solubility, dissolution rate, hygroscopicity, purity, bioavailability, should be included in the specification draft as much as possible. Detailed comparative data between the new crystal form and prior arts should also be provided. These may help to claim that the claimed invention improves multiple technical effects in a balance manner, or achieves unexpected technical effect in at least one or more aspects during prosecution or invalidation. In addition, other salt forms, or other crystal forms with worse technical effects obtained during the development of crystal forms may be described in the specification as well, to demonstrate that the claimed crystal form indeed achieves superior technical effects.
Furthermore, in the case that the law of the jurisdiction where the application filed allows supplement of experimental data after filing, even if there is no related data at the time of filing, describing multiple technical effects in the specification may also help to supplement experimental data in the subsequent proceedings.






