Exhaustion of Trademark Right cannot be Claimed for Repackaged Novartis Tablets, Ruled Taiwan Intellectual Property Court (NOVARTIS AG v Bramax Co., Ltd.)
Date: 10 May 2022
【Volume 79】
World-renowned pharmaceutical manufacturer, Novartis International AG, sells its products all around the world, and Taiwan is no exception. However, despite being such a huge multinational corporate, NOVARTIS still relies on numerous local wholesalers and retailers to reach its target consumers. Can local wholesalers and retailers freely use NOVARTIS’s registered trademarks in Taiwan? Is it legitimate for them to re-package the products to meet the needs of consumers?
According to the Taiwan Trademark Act, a trademark holder can no longer claim his trademark rights on a product after it has been put on the market with his consent. This provision is known as “the doctrine of exhaustion of trademark rights”. However, there is an exception: “Unless such claim is to prevent the condition of the goods from being changed or impaired after they have been put on the market or there exist other legitimate reasons”1 . In NOVARTIS AG v Bramax Co., Ltd. (Taiwan Intellectual Property Court 2018 Xingzhishangsuzi No. 44 Criminal Judgement), the Court clarifies the standard of the above-mentioned exception.
Case Fact
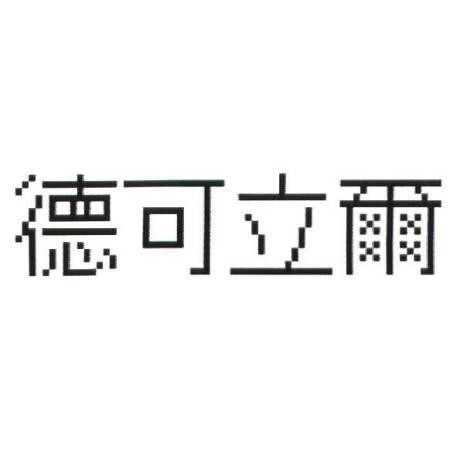
In 2011, NOVARTIS International AG (“NOVARTIS”) applied for the trademark “德可立爾” designating goods of class No. 5 in Taiwan. The application was granted registration in June 2012 (Registration No. 01519170, “the contested trademark”). NOVARTIS also began exporting goods with the contested trademark to Taiwan through its Taiwanese subsidiary, Novartis (Taiwan) Co., Ltd. (“NOVARTIS Taiwan”).
Bramax Co., Ltd. (“Bramax”, the defendant) had purchased “Coryol 40mg Film-coated Tablets (30 tablets per package)” from NOVARTIS Taiwan. However, the poor sales figures prompted Bramax to re-print the packaging boxes and tags with the contested trademark and sell them in boxes of 150 tablets each without the consent of NOVARTIS since November 2012.
Such act was revealed by the Public Health Bureau of Pingtung County Government in an audit in December 2012. NOVARTIS Taiwan then filed a criminal complaint against Peng, the head of Bramax. In October 2018, Taiwan Pingtung District Court convicted Peng of violating the Taiwan Trademark Act. Peng appealed to the Taiwan Intellectual Property Court (IP Court) and the Supreme Court successively. His appeal was rejected in 2020 by the Supreme Court, and the conviction was affirmed.
The contested trademark and the product bearing the contested trademark
| The contested trademark | The product bearing the contested trademark |
|---|---|
|
(Registration No. 01519170) |
Coryol 40mg Film-coated Tablets |
|
Class 5 Western medicine. |
The indication of this medication is: the adjuvant therapy of peripheral circulatory disturbance. It was manufactured in Germany and then imported to Taiwan by NOVARTIS Taiwan. |
Opinion of the IP Court
The IP Court pointed out that the purpose of Taiwan Trademark Act is protecting trademark rights and the interest of consumers, so it includes penalty for illegally selling products which violate trademark rights. However, legitimately selling products with trademarks will not cause likelihood of confusion over the quality or source of the products to the consumers. Oppositely, it leads to price competition and avoids certain trademark holders from monopolizing the market. Thus, it creates the benefit of free competition for relevant consumers who buy these products. Since such action does not contradict the purpose of statute, it does not constitute a violation of trademark rights either. On the other hand, if an action causes likelihood of confusion to consumers, it constitutes a violation of trademark rights and should be deemed guilty.
The court further exemplified the very situation which causes likelihood of confusion: “Using the trademark while selling without original packaging and processing, remodeling or altering the product without permission”. Furthermore, consumers must confuse the seller with the exclusive licensee, non-exclusive licensee, authorized agent, or authorized distributor of the trademark for the likelihood of confusion to be established. In this case, the court found that Peng had met the above-mentioned standard. Since his action had already caused confusion to consumers, he could not claim exhaustion of trademark rights.
Wisdom Suggested Strategies
Taiwan Trademark Act adopts the “doctrine of international exhaustion” which allows parallel imports. Consumers can buy products with trademarks and resell them without authorization, but they must be careful not to alter or process the original products nor take any action that may cause the consumers to confuse the resellers with the trademark holders or their authorized agents. Otherwise, they may still violate relating provisions in the Taiwan Trademark Act or Fair Trade Act.
The defendant in this case re-packaged the medication imported by NOVARTIS Taiwan and appointed an unknowing printing factory to print the contested trademark on the packaging boxes. While hearing this case, the IP Court indicated that such deed resulted in the consumers confusing the re-packaged medication with the original medication sold by NOVARTIS. Therefore, the defendant’s claim of trademark right exhaustion was not accepted by the court.
Those intending to import or resell products with others’ trademarks are suggested to evaluate local provisions concerning the exhaustion of trademark rights or parallel imports before beginning their commercial activities to protect their own interest and to avoid lengthy litigation.
[1] Paragraph 2, Article 36 of Taiwan Trademark Act