New Patent Linkage System in Taiwan: an effective solution to drug patent infringement disputes
Date: 30 December, 2020
【Volume 33】The patent linkage system in Taiwan took effect on 20 August, 2019 in accordance with the amended Pharmaceutical Affairs Act. The drug patent-approval linkage can expedite the issuance of generic drug permit, thus establishing an effective resolution mechanism for patent infringement disputes over a generic drug before the generic drug is brought to market.
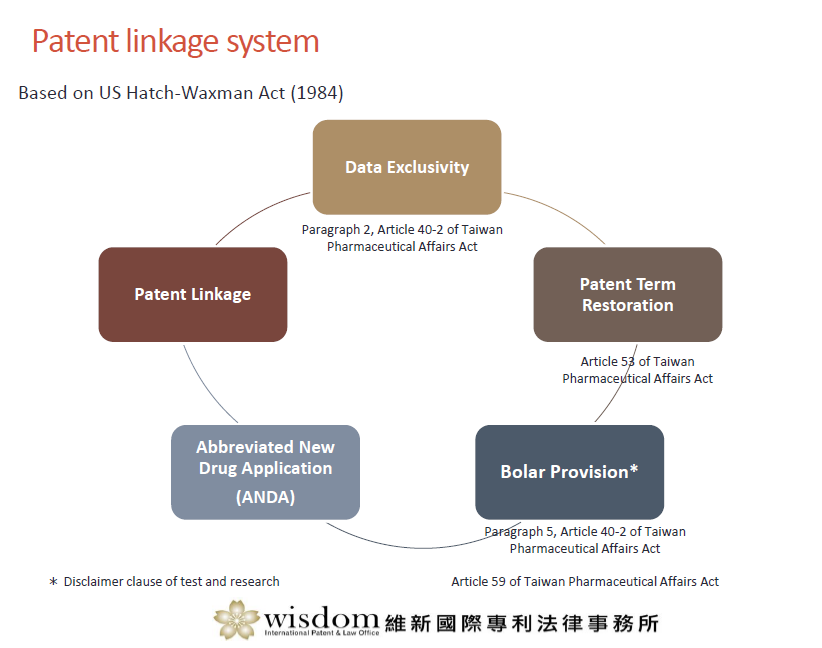
Patent Linkage System in Taiwan
The new patent linkage system in Taiwan is similar to the Hatch-Waxman Act governing US generic drug approvals.
1. Outline of the patent linkage system in Taiwan
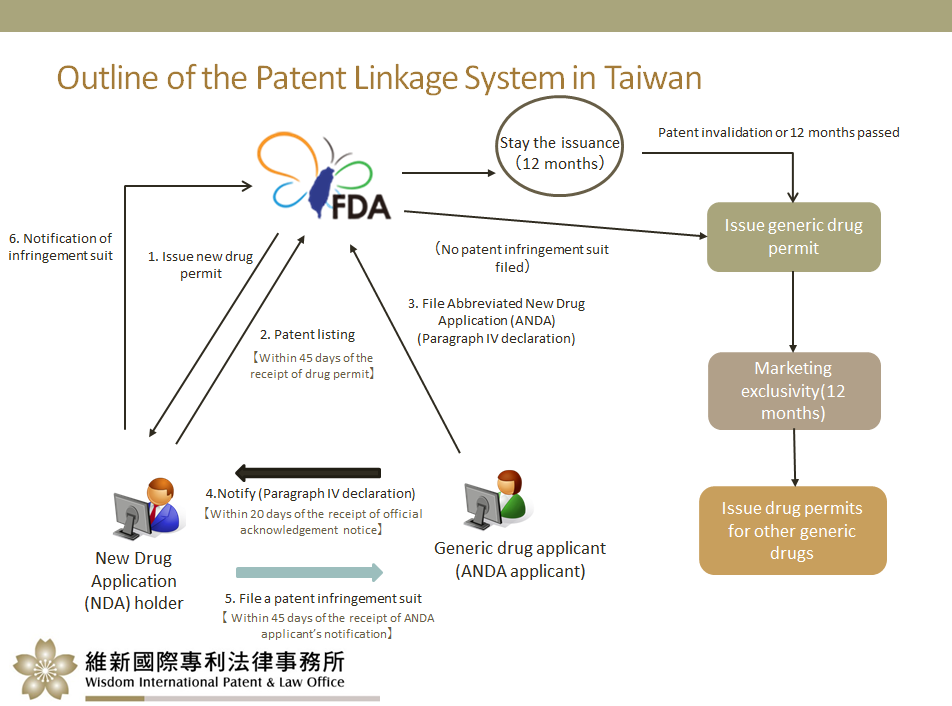
Under the new Act, generic drug manufacturers can make a Paragraph IV declaration (PIV declaration) when applying for a generic drug permit (similar to Abbreviated New Drug Application (ANDA) at the USFDA) with the Taiwan Food and Drug Administration (TFDA), i.e., stating that the corresponding patent rights of the new drug should be revoked, or that the generic drug applying for permit does not infringe on said rights.
An ANDA applicant must notify both the patentee and New Drug Application (NDA) holder of any Paragraph IV declaration. If the patentee initiates legal proceedings within 45 days, the TFDA will stay the issuance of the permit. Otherwise, the permit will be issued. If the patentee is unable to prevail in the lawsuit within 12 months, the first generic drug manufacturer shall be granted a 12-month exclusivity on the market.
2. Patent listing
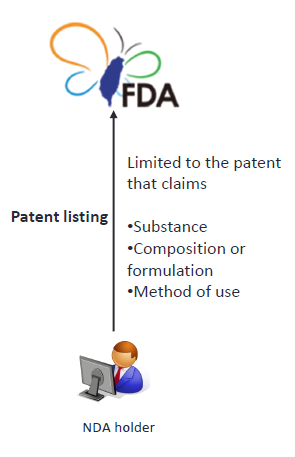 |
The NDA holder must submit the patent information regarding such drug to the TFDA within the stipulated period. (Article 48-3 of Taiwan Pharmaceutical Affairs Act) |
The NDA holder must submit a list of patent information regarding the new drug. The list shall include patents that claim the substance, composition or formulation, and medical use. The stipulated period is within 45 days after the next day to the receipt of the drug permit (mail delivered date).
Information submitted to the TFDA includes:
- Patent number (If the invention patent refers to medical use, the number of claims shall be provided);
- Patent expiry date;
- The patentee's name, nationality, place of domicile or business office; the name of the legal representative (for a patentee having a legal representative); and
- If said patent has been exclusively licensed, the aforementioned information of the exclusive licensee shall be listed.
If the holder of a new drug permit is different from the patentee, the patentee’s consent shall be obtained; if the patent has been exclusively licensed, it is only required to obtain the exclusive licensee's consent.
3. Update of patent information
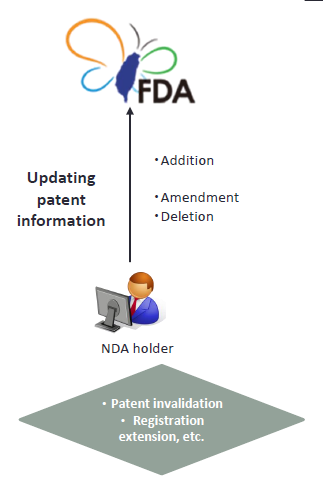 |
If the NDA holder obtains the approval of an invention patent after the approval of a new drug permit, and such patent is subject to the scope of drug patent set forth in Paragraph 2 of Article 48-3, the patent information thereof shall be submitted within the stipulated period.(Article 48-5 of Pharmaceutical Affairs Act)
The NDA holder shall amend or delete the listed patent information within the stipulated period in the following matter.(Article 48-6 of Pharmaceutical Affairs Act) |
Addition of patent information:
- Stipulated period: Within 45 days after the next day to the patent issuance
- Listing information: in accordance with Article 48-4
Amendment/Deletion of patent information:
- Stipulated period: Within 45 days after the next day to the occurrence
- Conditions:
- The patent term extension is approved and issued
- The post-grant amendment to patent claim(s) is approved and issued
- The patent has been revoked
- The patent has become extinguished
- Amendment of name, address, etc.
4. Patent information examination, publication, and notification by the third party
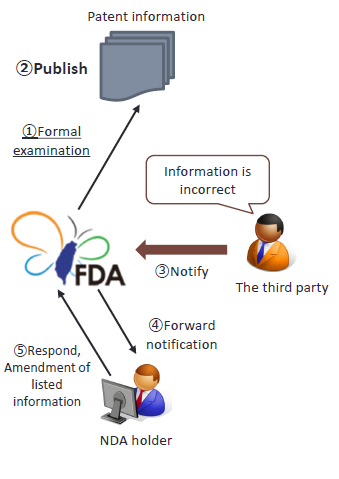 |
The TFDA shall publish the patent information, the third party's allegations and the written responses made by the NDA holder.(Article 48-8 of Pharmaceutical Affairs Act)
The NDA holder shall amend or delete the listed patent information within the stipulated period in the following matter.(Article 48-6 of Pharmaceutical Affairs Act) |
Anyone may notify the TFDA in the occurrence of the following matters:
- The invention listed in the patent information is irrelevant to the approved drug;
- The invention listed in the patent information is not substance, composition or formulation, or medical use;
- The patent information listed is incorrect;
- No amendment or deletion has been made for any of the changes in the patent.
The TFDA shall, within 20 days after the next day to its receipt of the notification, forward said notification to the holder of the new drug permit.
The NDA holder shall, within 45 days of the next day to its receipt of said notification, respond with written explanations, and may amend or delete the patent information as the case may be. There will be penalties if no response is submitted within the stipulated period.
5. Patent declaration for ANDA
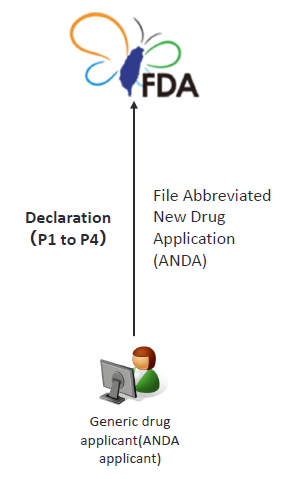 |
The ANDA applicant shall, with respect to each patent or claim of the approved new drug listed by NDA holder, declare one of the following items.(Article 48-9 of Pharmaceutical Affairs Act) |
The ANDA applicant shall declare one of the following items:
- No patent information of said new drug has been listed;
- The patent corresponding to said new drug has extinguished;
- The TFDA will issue the generic drug permit after the patent corresponding to said new drug extinguishes; or
- The patent corresponding to said new drug shall be revoked, or the patent corresponding to said new drug will not be infringed by the generic drug subject to the application for drug permit. (Paragraph IV declaration).
The corresponding patent is not limited to one, and a declaration shall be made for each corresponding patent.
6. Notification by generic drug applicant after PIV declaration
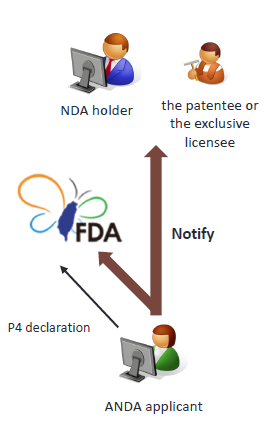 |
When PIV is declared, the ANDA applicant shall notify the NDA holder and the TFDA with written notification within the stipulated period. (Article 48-12 of Pharmaceutical Affairs Act) |
The stipulated period is within 20 days after the next day to its receipt of official acknowledgement notice that all ANDA documents have duly prepared.
In the written notification, the ANDA applicant shall provide an explanation and evidence regarding its allegation that the patent shall be revoked or that there is no patent infringement. It is not necessary to state the trade secret or the attack defense method adopted in the upcoming lawsuit.
The TFDA shall dismiss the ANDA if the applicant fails to issue the notification or the notification does not meet the requirements.
7. Litigation and stay
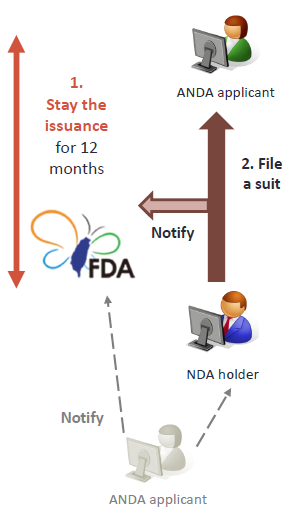 |
Upon receipt of the notification from ANDA applicant, the NDA holder, patentee or the exclusive licensee can file a patent infringement suit and notify the TFDA. The TFDA shall stay the issuance of the drug permit of ANDA applicant after notification.(Article 48-13 of Pharmaceutical Affairs Act)
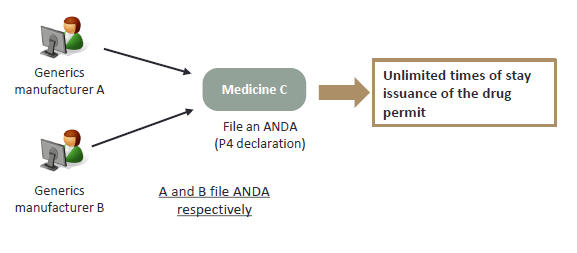
For the applications for the generic drug permits filed by the same applicant for the same drug, the TFDA may only stay the issuance of the drug permit in accordance with the preceding Article once. (Article 48-14 of Pharmaceutical Affairs Act) |
- The TFDA shall stay the issuance of the drug permit for 12 months as of the next day to the receipt of the ANDA applicant’s notification to the NDA holder
- File a patent infringement suit:
The patentee or the exclusive licensee shall file a lawsuit within 45 days after the next day to the receipt of notification.(If both receive the notification, the period shall commence on the later date.)
The patentee may choose to initiate civil infringement litigation (infringement removal, infringement prevention). If the patentee or the exclusive licensee fails to file a suit within 45 days after receiving notification, the ANDA applicant may file a declaratory judgment as to confirm whether the generic dug causes infringement.
For the same ANDA filed by the same applicant based on different patents, stay issuance of the drug permit multiple times is not allowed.
8. Issuance of generic drug permit
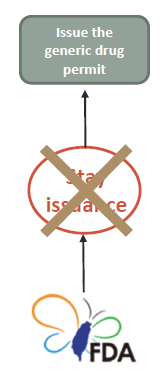 |
In any of the following matters, the TFDA is not subject to the regulation set forth in the preceding paragraph. The generic drug permit can be issued subject to passing examination and meeting the provisions of this Act and other regulations. (Article 48-13 of Pharmaceutical Affairs Act) |
If there is any of the following matter, the TFDA may issue the generic drug permit if the ANDA is examined to be in compliance with the regulations under this Act:
- The patentee or exclusive licensee fails to file a suit within the stipulated period;
- The patentee or the exclusive licensee files a suit based on the patents which are not those listed before the date of the application for the generic drug permit;
- After filing a suit, the plaintiff’s complaint is overruled by the court;
- The decision of infringement has not been made within 12 months after the suit filed (if the final judgment confirming infringement is made within 12 months after the suit filed, the generic drug permit cannot be issued);
- The court has determined that the all of the patents pending in the infringement lawsuit shall be revoked, or a non-infringement judgment is obtained by the ANDA applicant;
- The Intellectual Property Office makes an invalidation decision regarding all the patents or claims under the PIV declaration;
- A settlement or mediation has been reached by the parties
(Within 20 days after the next day to the occurrence of any settlement agreement or arbitration agreement, both parties shall notify the TFDA and the Fair Trade Commission. Article 48-19);
- All the patents or claims under the PIV declaration have become extinguished.
9. Marketing exclusivity
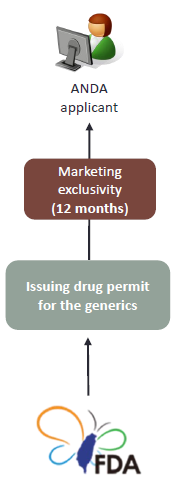 |
The application for a generic drug permit with application documents duly prepared at the earliest shall be granted a 12-month period of marketing exclusivity. (Article 48-16 of Pharmaceutical Affairs Act) |
The marketing exclusivity period which starts from the date of the actual marketing of the generic drug, will be given to the first ANDA applicant. The TFDA shall not issue other drug permits to other generics before the expiration of the 12-month period.
Determination of the “first ANDA Applicant”?
The ANDA under PIV declaration with application documents duly prepared at the earliest.
If more than one ANDA has the same date of duly prepared application documents, such applications are jointly subject to the marketing exclusivity period.
In this case, the commencement date shall be the date on which any of such drugs are actually first marketed.
In any of the following matters, the vacancy for marketing exclusivity period will be fulfilled by the subsequent applicant with application documents duly prepared:
- During the period of drug permit examination, the declaration under PIV is amended;
- The earliest applicant fails to obtain from the TFDA the notification that the examination of ANDA has completed within 12 months after the next day to the date that all the application documents are duly prepared;
- During the stay issuance of the drug permit, final infringement decision is made.
10. NDA holder before the enforcement of the amended provisions
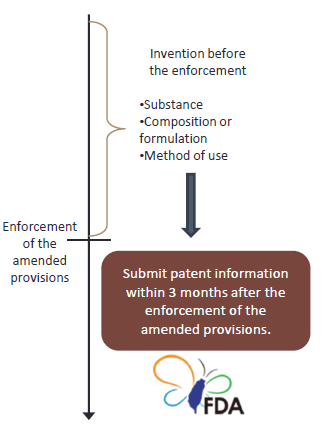 |
Before the enforcement of the amended provisions, the holder of a new drug permit whose drug patent(s) is subject to the drug patent as stipulated in Paragraph 2, Article 48-3, may submit patent information within 3 months after the enforcement of the amended provisions.(Article 48-21 of Pharmaceutical Affairs Act) |
This provision is limited to the drug patent that has not become extinguished yet. It is not an obligation to submit patent information to the TFDA.
11. Regulations for data exclusivity in Taiwan
New drug of a new molecular entity (Article 40-2 of Pharmaceutical Affairs Act)
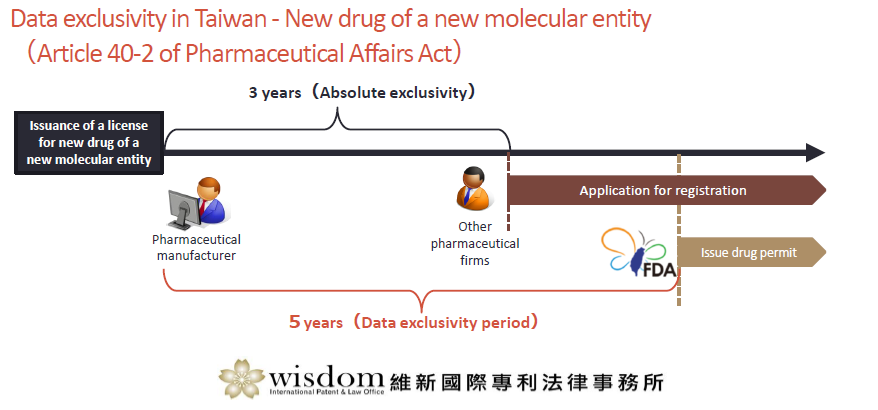
Within 3 years after the issuance of a license for new drug of a new molecular entity, no other pharmaceutical firm may apply for registration of the same drug by citing the application data submitted by said drug permit holder without such holder’s consent.
The TFDA may issue a drug permit to other pharmaceutical firms only after the next day to the expiration of the 5-year period after the issuance of said new drug permit of new molecular entity.
The 3-year absolute exclusivity period is applicable only when a registration application is filed with the TFDA within 3 years after marketing approval is obtained in any country.
New indications (Article 40-3 of Pharmaceutical Affairs Act)
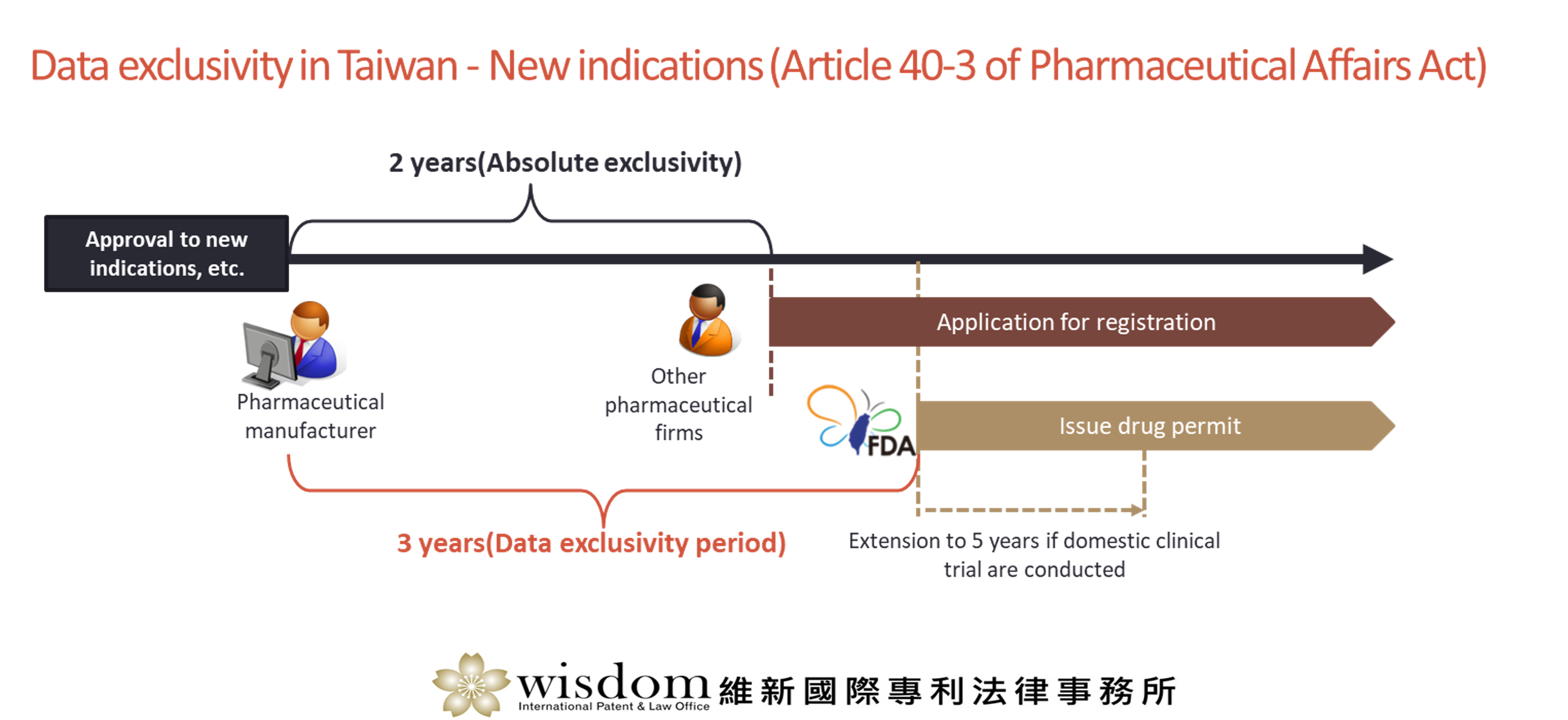
For a drug that has been approved by the TFDA to supplement or amend the indications thereof, within 2 years after the approval of such supplements or amendments to indications, no other pharmaceutical firm may apply for registration of the same indication by citing the application data submitted by said drug permit holder without such holder’s consent.
The TFDA may issue drug permit to other pharmaceutical firms only after the next day to the expiration of the 3-year period after the approval of supplements or amendments to indications. However, if the domestic clinical trials regarding such supplements or amendments to indications are conducted, the 3-year period may be extended to 5 years.
This Article is applicable only when an application for registration is filed with the TFDA within 2 years after marketing approval is obtained in any country.
Our patent team can handle all aspects of obtaining and protecting a patent, from drafting the patent specification and responding to the Office Actions, to handling appeals and litigations before the Taiwan IP Office and the courts. With the extensive experiences of our patent attorneys, we are capable to give you the most secure protection on your patents and intellectual property rights. Contact us if you wish to find out more about IP protection.






