Top 10 Patent Cases of 2021 - Actelion’s OPSUMIT® Pharma Patent Survived the Invalidation
Date: 28 July 2022
The patentee of "Novel Sulfamides and Their Use as Endothelin Receptor Antagonists” (CN01820481.3) is Actelion Pharmaceuticals Ltd. The patent at issue relates to a compound/pharmaceutical composition/use of a brand-name drug called Macitentan (OPSUMIT®). The medicine has been approved as the first oral formulation for the treatment of pulmonary arterial hypertension. After the submission of generic drug registration for Macitentan, Nanjing Chia-Tai Tianqing Pharmaceutical Company filed a patent invalidation request. The China National Intellectual Property Administration (CNIPA) issued Patent Invalidation Decision No. 48183 in 2021, which upheld the validity of the patent based on the patentee’s post-grant amended specification.
The CNIPA made a classic decision regarding three main concerns, which were: Markush structure as a priority, sufficiency of disclosure, and inventiveness of the compounds. The case was selected as one of the "Top 10 Patent Reexamination and Invalidation Cases of 2021."
The Main Technical Features of the Patent at Issue
The patentee amended the claims during the invalidation trial, deleting claims 1~10 and all but two specific compounds (i.e., compound 104 and Macitentan) in claim 11. After amendment, claim 11 became the new claim 1.
Claims 1~8 after post-grant amendment:
Claim 1 refers to a compound, which had been narrowed down to two specific compounds, namely, compound 104 and Macitentan.
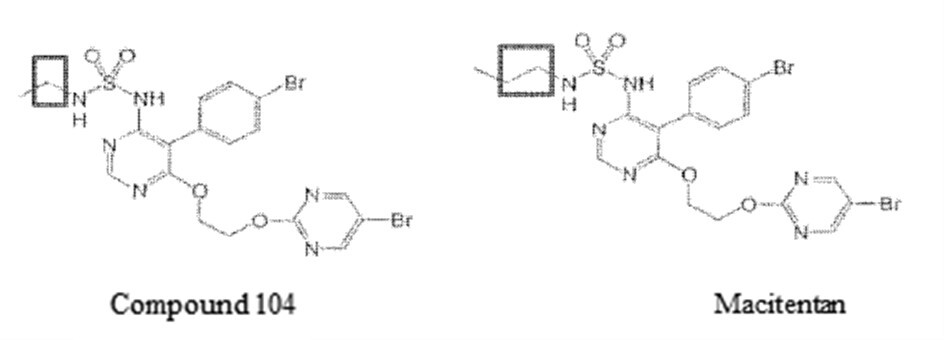
Claims 2~8 refer to pharmaceutical compositions and uses comprising the compound of claim 1.
Decision of the CNIPA
I.Markush structure as a priority
According to Article 29 of the Patent Law of China, "an invention of the same subject" enjoys the right of priority of the first foreign application. However, if the latter application comprises a species of a Markush structure of the former application, which does not specify the particular compound, and a PHOSITA cannot confirm that it is included in the former application based on its overall content, the priority cannot be claimed.
Exhibit 2 (the priority document) does not provide the name, structure, preparation, or experimental data for the effect of compound 104 and Macitentan, as may be seen by comparing it to the patent at issue. Considering that compound 104 and Macitentan of the amended claim 1 were not specified in the priority document, the patent at issue cannot claim priority of Exhibit 2.
II. Sufficiency of disclosure concerning the compounds listed in the form of a table
For compound 104, the specification reveals its LC-MS data and IC50 value for ETA and ETB, and yet its preparation method is not reported. As for Macitentan, the specification only provides its structure.
i. Whether the disclosure of compound 104 is complete: Despite not having disclosed the method for preparing compound 104 in the specification, a PHOSITA would nonetheless be able to produce the compound.
The specification disclosed the structure and LC-MS data of compound 104, as well as its antagonistic activity on ETA and ETB. Accordingly, a PHOSITA would be able to validate that the patentee had obtained the compound by the filing date of the patent at issue.
In addition, a PHOSITA would be able to synthesize compound 104 based on the examples given in the specification. For one thing, according to the explicit description on page 66, compound 15-202, which includes compound 104, can be prepared by following examples 1-14. For another, synthesizing compounds of the kind requires 5 key steps as specified by the examples. And the intermediates of the first 3 steps of synthesizing compound 104 have been disclosed:
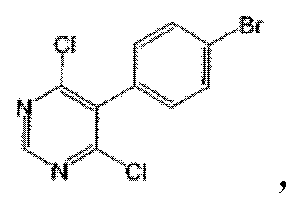
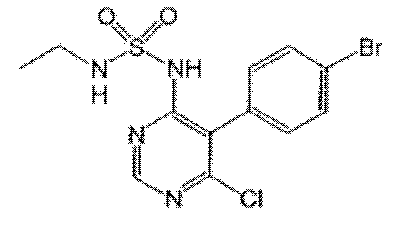
Although the intermediate from step 4 is not disclosed in the specification, a PHOSITA can anticipate the outcome of the said step due to the fact that the chlorine substitution at position 6 of the pyrimidine is more reactive than that of the bromine of the bromobenzene at position 5 of the pyrimidine.
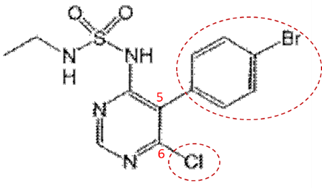
ii. Whether the disclosure of Macitentan is complete: The specification provides the structure of the compound but lacks examples of its preparation and effect. Still, it does not imply that the compound invention is not completed.
The structural difference between compound 104 and Macitentan lies in the alkyl chain connecting to the sulfamide. They are an ethyl group and a propyl group, respectively (shown in the following figure).
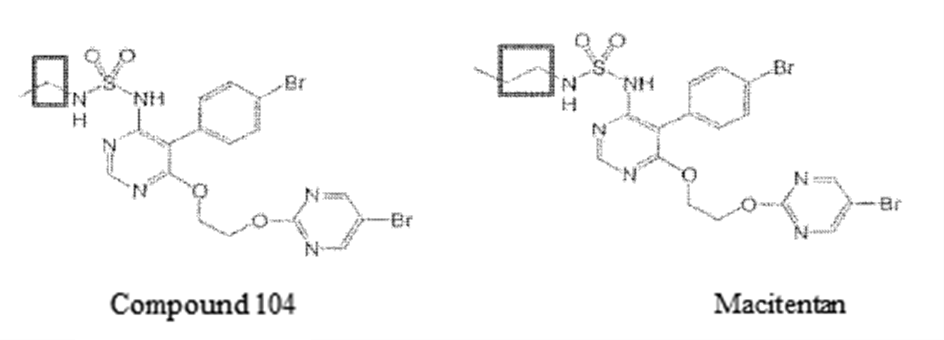
The alkyl chain is introduced into the compound via the reaction between an alkyl amine and a sulfamoyl chloride. It is of no technical difficulty for a PHOSITA to replace the ethylamine used to produce compound 104 with propylamine to prepare Macitentan, and the outcome can be reasonably expected. Moreover, replacements as such have been disclosed in the specification. For example, compounds 115 and 117 have the following structure:
wherein the R1 for compounds 115 and 117 is, respectively, an ethyl group and a butyl group. Similar to compound 104 and Macitentan, the difference between compounds 115 and 117 lies in that the alkyl group is individually an ethyl and a butyl. When there is no technical difficulty with the introduction of ethyl and butyl, there is no reason for a PHOSITA to doubt the possibility of introducing propyl in a similar manner and thus obtain Macitentan. Consequently, taking the overall description in the specification into account, a PHOSITA would be able to confirm the structure of Macitentan and produce it. Also, since compound 104 and Macitentan are alike in structure as they are merely a methylene apart, a PHOSITA can reasonably expect that Macitentan possesses similar technical effects as compound 104.
III. Inventiveness of the compounds
i. Distinct Technical Feature
The distinct technical features between compounds 104 and Macitentan of the patent at issue and compound 7k of Exhibit 5 are: (1) the substitution at position 5 of the pyrimidine ring; (2) the sulfamide moiety at position 4 of the pyrimidine ring. It is an ethylamine (compound 104) or a propylamine (Macitentan) that connects to the sulfone of sulfamide for the patent at issue, while a tert-butylbenzene for Exhibit 5.
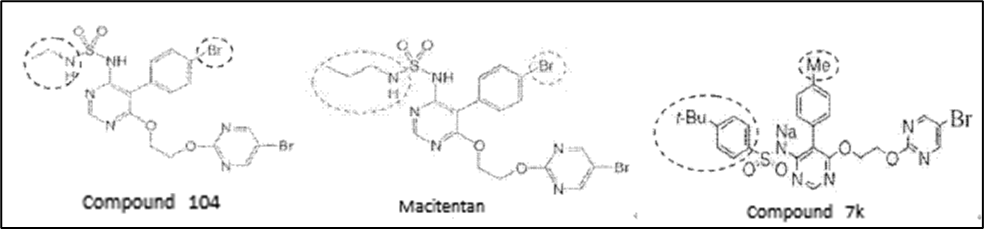
ii. Technical Problems to be Solved
Compared to Exhibit 5, the patent at issue intends to provide a compound that has a different structure and is antagonistic to both ETA and ETB receptors. The key to determining inventiveness falls on whether there is motivation to incorporate the aforementioned distinct technical features into the patent at issue based on Exhibits 6, 7, and 9 set forth by the petitioner and also Exhibits 16, 22, and 23 that demonstrate the common general knowledge of the art.
iii. Obviousness
Exhibit 6 is a structure-activity study based on Bosentan (as shown below). The study focuses on how the substitutions at positions 4 and 6 of pyrimidine affect the endothelin receptor. With varying substitutions connecting to the sulfamide moiety at position 4, compounds 6e, 6p, 6q, and 6r can be obtained. And yet, the compounds disclosed in Exhibit 6 all relate to a carbon connecting to the sulfamide, and the same applies to Exhibit 7. Therefore, as per the teaching of Exhibit 6 and/or 7, a PHOSITA would have no motivation to modify and replace the carbon connecting to the sulfamide (-C-SO2-NH-) as in Exhibit 5 with a nitrogen (-NH-SO2-NH-).
Furthermore, Exhibits 16, 22, and 23 contain general introduction of bioisostere, which does not involve the endothelin receptor antagonist of the patent at issue. Exhibits 16, 22, and 23 alone do not prove that a PHOSITA would be motivated to replace the carbon connecting to the sulfamide with a nitrogen on the basis of bioisostere since the substitutions shown in Exhibit 6 tend to retain the carbon connecting to the sulfamide and alter other parts.
Additionally, the main structures of the compounds in Exhibit 9 are a pyrrolidone and a pyrazole acid, which completely differ from the pyrimidine ring of the patent at issue. Thus, a PHOSITA would not have been inspired to replace the tert-butylbenzene that connects to the sulfamide at position 4 of the pyrimidine (as in evidence 5) with an ethylamine or a propylamine.
In conclusion, even though a PHOSITA would have been motivated to include distinct feature (1) based on Exhibit 5 and modify the structure of compound 7k, the motivation to incorporate distinct feature (2) is still lacking. As a result, the patent at issue is ruled to be inventive.
Wisdom Suggested Strategies
When it comes to determining the inventiveness of a compound, two factors should be taken into consideration. One is the obviousness of the structure of the compound, and the other is the obviousness of the pharmacological effect of the compound. After the issuance of the second instance judgment regarding the invalidation case of the compound Ticagrelor, we can see in the ensuing invalidation cases (e.g., Rivaroxaban case (Decision No. 45997), Alogliptin case (Decision No. 48855), Macitentan case, etc.) that the CNIPA is beginning to place more emphasis on the structure-activity relationship of the prior art compound based on the overall teaching of the prior art and judge the obviousness of the compound at issue accordingly. Patentees and petitioners are advised to stay up to date with the trend.
For more information on this topic or if you have any questions, please contact Wisdom by email (info@wisdomlaw.com.tw) or phone (+886-2-2508-2466 ext. 220). Click here to subscribe to our Wisdom News and stay updated on the latest intellectual property developments.






