IP Court Establishes Selection of Lead Compound and Motivation to Modify the Lead Compound | Millennium Pharm., Takeda Pharm. v. Chunghwa
Date: 19 May 2022
Compound patent may be in the earliest stage in the development of a pharmaceutical patent portfolio, and thus is the core of pharmaceutical patents. Taiwan Patent Examination Guidelines do not explicitly stipulate the motivation for modifying the structure of compounds. However, it is worth noting that Taiwan Intellectual Property Court (IP Court) ruled in the recent case of Millennium Pharmaceuticals, Inc., Takeda Pharmaceuticals Taiwan Ltd. v. Chunghwa Compound Synthesis & Biotech Co., Ltd. (2021 MinZhuanSuZi No.19 Judgment), and established the standards of“selection of lead compound” and “motivation to modify the lead compound”. In this three-part series, we will introduce and analyze the case, and further compare the differences between the examination practice in Taiwan, the United States, Europe, and China.
I. Millennium Pharmaceuticals, Inc., Takeda Pharmaceuticals Taiwan Ltd. v. Chunghwa Compound Synthesis & Biotech Co., Ltd. (Taiwan IP Court 2021 MinZhuanSuZi No.19 Judgment)
Case Fact
Millennium Pharmaceuticals, Inc. (patentee, plaintiff of the civil litigation) is the patentee of Invention Patents “PROTEASOME INHIBITORS” (Certificate No.: I440641) (“‘641 patent”), “BORONATE ESTER COMPOUNDS AND PHARMACEUTICAL COMPOSITIONS THEREOF” (Certificate No.: I498333) (“‘333 patent”) and “BORONATE ESTER COMPOUNDS AND PHARMACEUTICAL COMPOSITIONS THEREOF” (Certificate No.: I543985) (“‘985 patent”), and Takeda Pharmaceuticals Taiwan Ltd. (plaintiff of the civil litigation) is the exclusive licensee of ‘641 patent. The plaintiffs filed a lawsuit and claimed that Chunghwa Compound Synthesis & Biotech Co., Ltd. (defendant) applying for drug permit of “Ixazomib Citrate” (drug at issue) to TFDA has infringed the ‘641 patent, ‘333 patent and ‘985 patent. However, the court deemed ‘641 patent shall be revoked and thus the drug at issue did not infringe the ‘641 patent, while it has infringed the ‘333 patent and ‘985 patent.
Main technical features of the patents at issue
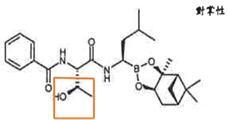
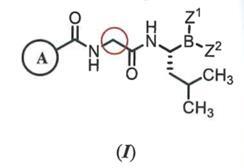
(1) Compound (I) of Claim 1 of the ‘641 patent:
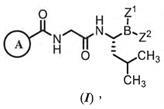
…wherein: Z1 and Z2 are each independently…; or Z1 and Z2 together form a moiety derived from a boronic acid complexing agent; and Ring A is selected from the group consisting of phenyl groups substituted by 1-2 Cl or F atom(s).
Arguments of the Plaintiffs
According to Table F-1 of Citation 1 (Taiwan Patent No. 200529810), about 143 compounds exhibit both best inhibitory activity against HEP and MOLT4 (both classified as +++), while compound D.3.174 shows “+++(EC50 value below 200 nM)” against MOLT4 and merely “++(IC50 value below 100 nM) against HEP, which is not the one having best inhibitory activity against HEP (+++). Therefore, lead compound shall be selected from the 143 compounds having both best inhibitory activities against HEP and MOLT4, and there exists no motivation or reason to select compound D.3.174, which does not have the best inhibitory activity, as the lead compound to be further developed.
| Compound D.3.174 of Citation 1 | Compound (I) of the ‘641 patent |
|---|---|
|
|
|
Opinion of the IP Court
The IP Court deemed the ‘641 patent is obvious in view of Citation 1:
(1) Based on the compounds disclosed in Citation 1, a skilled person is reasonably motivated to select compound D.3.174 as lead compound, and can easily accomplish the compound (I) of ‘641 patent by simple modification through routine experiments in accordance with the teachings of Citation 1.
To determine whether there exists a “reasonable motivation” for a skilled person to make the compound (I) of ‘641 patent based on the compounds disclosed by the Citation 1 through routine works, besides structural similarity between claimed compound and prior art compounds, it is also necessary to consider whether the two have similar pharmacological activities or treat similar diseases. Hindsight bias is likely to occur if only the structural similarity is met without similarity in pharmacological activities. Hence, according to the results regarding the pharmacological activity data of the compounds in the Example of Citation 1, one should assess the relation between the structure and its effects, and then select a lead compound with inhibitory activity against both HEP and MOLT4 as the starting point for comparing structural similarity. Since the compound D.3.174 of Citation 1 shows “+++” and “++” in the test regarding EC50 of MOLT4 cells and IC50 of HEP, respectively, which demonstrates its inhibitory activity against HEP and MOLT4, a skilled person is reasonably motivated to select compound D.3.174 as lead compound. In addition, in terms of the chemical structural differences between compound D.3.174 and compound (I) of the ‘641 patent, Citation 1 also teaches the modification directions of lead compound. Accordingly, based on the disclosure of Citation 1, a skilled person can easily obtain compound (I) of the ‘641 patent by simple modification through routine experiments.
(2) Compounds selected as lead compounds are not necessary to have the most excellent inhibitory effects. A compound, which is clearly disclosed and listed as active by Citation 1, is deemed potential and qualifies as one of the candidates for lead compounds.
The plaintiffs claimed that compound D.3.174 does not have the best HEP inhibitory effect, which is not the 143 compounds with both the best HEP and MOLT4 inhibitory activities disclosed in Citation 1, and thus a skilled person should select a lead compound from the 143 compounds without motivation to select compound D.3.174. However, such argument was not recognized by the court. The court held that according to the activity data described in Citation 1, a compound is deemed active as long as its HEP (IC 50) value and MOLT4 (EC50) value meets “+” level, and thus is a potential compound in Citation 1, which reasonably motivates a skilled person to select as a lead compound for further research. Furthermore, since the HEP test result of compound D.3.174 is not the best, its activity still has room for improvement. Therefore, a skilled person is more motivated to select it as a lead compound for further optimization research to synthesize an improved compound with better inhibitory activity. Accordingly, the plaintiffs’ claim that only the compound with both the best inhibitory effects in HEP and MOLT4 assays can be selected as a lead compound is meritless.
Wisdom Suggested Strategies
Taiwan Patent Examination Guidelines do not explicitly stipulate the motivation for modifying the structure of compounds. Whether a compound involves an inventive step is determined primarily by structural similarity. If the claimed compound does not resemble the prior art compound, the claimed compound involves an inventive step. If the compounds are similar and have similar uses, in principle, the claimed compound does not involve an inventive step unless proven to have unexpected effects. This standard is the same as in China. Pertaining to structural similarity, the Patent Examination Guidelines state that molecular structure alone cannot determine the structural similarity between two compounds; other aspects such as the technical field, relevance of the structure and effect, scope of application, and so on must be considered.1
In this case, the IP court ruled that lead compound selection must be “similar in terms of structure as well as pharmacological action”. Hindsight bias is likely to occur if only structural similarity but not pharmacological action is taken into consideration. Moreover, the judgment specifically stated that "compounds which are selected as the lead compounds with reasonable motivation do not necessarily have the best effect". Analyzing the judgement trends on the inventive steps in pharmaceutical cases in Taiwan in recent years, the Taiwan IP court tends to pay more attention to the judgement criteria in US. Accordingly, patentees or invalidation petitioners can attempt to adopt US judgement criteria in their arguments to improve the chance to win.
[1] Taiwan Patent Examination Guidelines Part II, chapter 13